Amino Acid δ13C Analysis Shows Flexibility in the Routing of
... tissues both of consumers and of their potential prey be lipid-extracted prior to analysis, because (1) lipids have carbon isotope (d13C) values that are lower by approximately 3–8ø than associated proteins and (2) amino acids in consumers’ proteinaceous tissues are assumed to be completely routed f ...
... tissues both of consumers and of their potential prey be lipid-extracted prior to analysis, because (1) lipids have carbon isotope (d13C) values that are lower by approximately 3–8ø than associated proteins and (2) amino acids in consumers’ proteinaceous tissues are assumed to be completely routed f ...
1 Protonolysis of Fe-C bonds of a Diiminopyridineiron(II) Dialkyl
... 4.2. General procedure for the reaction of 1 with protic acids in stoichiometric ratio 1:1. A solution containing approx. 440 mg of compound 1 (0.7 mmol) in 40 ml of THF was stirred at -80 ºC, and a second solution containing exactly the equimolar amount of the corresponding acid (HY = perfluorophen ...
... 4.2. General procedure for the reaction of 1 with protic acids in stoichiometric ratio 1:1. A solution containing approx. 440 mg of compound 1 (0.7 mmol) in 40 ml of THF was stirred at -80 ºC, and a second solution containing exactly the equimolar amount of the corresponding acid (HY = perfluorophen ...
Mastering the California Science Content Standards, SE
... investigations. As a basis for understanding this concept and addressing the content in the other three strands, students should develop their own questions and perform investigations. Students will: a. Plan and conduct a scientific investigation to test a hypothesis. b. Evaluate the accuracy and re ...
... investigations. As a basis for understanding this concept and addressing the content in the other three strands, students should develop their own questions and perform investigations. Students will: a. Plan and conduct a scientific investigation to test a hypothesis. b. Evaluate the accuracy and re ...
Development of a novel analytical approach combining the quantification of
... pyruvic. Simultaneously, we take advantage of mass spectrometry and NMR as complementary techniques to LC. In that respect, due to the complexity of isolation of interferences such as hyaluronic acid (HA, an anionic, non-sulfated glycosaminoglycan) present in the culture media, glucose required the ...
... pyruvic. Simultaneously, we take advantage of mass spectrometry and NMR as complementary techniques to LC. In that respect, due to the complexity of isolation of interferences such as hyaluronic acid (HA, an anionic, non-sulfated glycosaminoglycan) present in the culture media, glucose required the ...
Evidence for the Predominance of Condensed Phase Reaction in
... and Fe2O3, respectively. A typical background spectrum for the mass spectrometer consists of a large peak at mass to charge ratio (m/z) 18 (H2O) and smaller peaks at m/z 28 (N2), m/z 32 (O2), and m/z 17 (OH), which results from fragmentation of H2O during ionization. The main reaction product for th ...
... and Fe2O3, respectively. A typical background spectrum for the mass spectrometer consists of a large peak at mass to charge ratio (m/z) 18 (H2O) and smaller peaks at m/z 28 (N2), m/z 32 (O2), and m/z 17 (OH), which results from fragmentation of H2O during ionization. The main reaction product for th ...
Old Exam
... Carbon has two common isotopes 12C and 13C. 12C has a relative abundance 98.892% and one atom has an exact mass of 12.0000 u. 13C has a relative abundance of 1.108% and one atom has an exact mass of 13.0034 u. a. (3 pts) How many protons, neutrons and electrons in an atom of 12C? ...
... Carbon has two common isotopes 12C and 13C. 12C has a relative abundance 98.892% and one atom has an exact mass of 12.0000 u. 13C has a relative abundance of 1.108% and one atom has an exact mass of 13.0034 u. a. (3 pts) How many protons, neutrons and electrons in an atom of 12C? ...
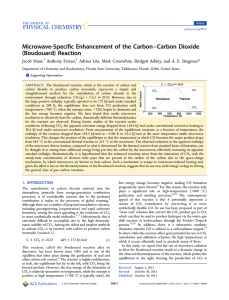
Microwave-Specific Enhancement of the Carbon−Carbon Dioxide
... the free energy becomes negative. We have found that, under microwave irradiation to selectively heat the carbon, dramatically different thermodynamics for the reaction are observed. During kinetic studies of the reaction under conditions of flowing CO2, the apparent activation energy dropped from 118 ...
... the free energy becomes negative. We have found that, under microwave irradiation to selectively heat the carbon, dramatically different thermodynamics for the reaction are observed. During kinetic studies of the reaction under conditions of flowing CO2, the apparent activation energy dropped from 118 ...
1.1 Functional Groups of Biomolecules and their Reactions
... if equal substituents are on the same side and a trans-configuration if they are on opposite sides (Fig. 1.1.2). This type of isomerism is particularly relevant for the physical properties of compounds, with differences being greater if polar groups are present. Reactivity is also affected, as cis c ...
... if equal substituents are on the same side and a trans-configuration if they are on opposite sides (Fig. 1.1.2). This type of isomerism is particularly relevant for the physical properties of compounds, with differences being greater if polar groups are present. Reactivity is also affected, as cis c ...
day_3_main_lecture - the Essentially Science Wiki!
... Activity – Determine the Molar Mass of Butane Review safety concerns and protocols !!!!! 1. Identify at least 4 possible sources of experimental error 2. The accepted molar mass of butane is 58.0 g/mole. Determine percentage error for your data. 3. Use your data, assume a molar mass of 58.0 g/mol, ...
... Activity – Determine the Molar Mass of Butane Review safety concerns and protocols !!!!! 1. Identify at least 4 possible sources of experimental error 2. The accepted molar mass of butane is 58.0 g/mole. Determine percentage error for your data. 3. Use your data, assume a molar mass of 58.0 g/mol, ...
CARBANIONS Carbanions are units that contain a negative charge
... The stabilizing dispersal of the electrons into the EWG is shown in the examples below. Carbonyl functions are very effective in stabilizing adjacent negative charge and when two carbonyl groups are present (as in diethyl malonate or acetylacetone) a very useful carbanionic intermediate is produced. ...
... The stabilizing dispersal of the electrons into the EWG is shown in the examples below. Carbonyl functions are very effective in stabilizing adjacent negative charge and when two carbonyl groups are present (as in diethyl malonate or acetylacetone) a very useful carbanionic intermediate is produced. ...
2 - AQA
... to use helium as its lifting gas, rather than hydrogen, but the only source of large volumes of helium was the USA and they refused to sell it to Germany because of Hitler’s aggressive policies. The airship was therefore made to use hydrogen. It held about 210 000 m3 of hydrogen gas but this volume ...
... to use helium as its lifting gas, rather than hydrogen, but the only source of large volumes of helium was the USA and they refused to sell it to Germany because of Hitler’s aggressive policies. The airship was therefore made to use hydrogen. It held about 210 000 m3 of hydrogen gas but this volume ...
Exam 4 - Chemistry Courses
... D. The equilibrium partial pressure of BrCl(g) will be greater than 2.00 atm. E. The reaction will go to completion since there are equal amounts of Br2(g) and Cl2(g). ...
... D. The equilibrium partial pressure of BrCl(g) will be greater than 2.00 atm. E. The reaction will go to completion since there are equal amounts of Br2(g) and Cl2(g). ...
Atomic Mass: The atomic mass of an element is the mass average of
... Chemical equation • When a chemical reaction occurs, it can be described by an equation. • This shows the chemicals that react (reactants) on the left-hand side, and the chemicals that they produce (products) on the righthand side. Reaction conditions Reactants Products Reaction between hydrogen gas ...
... Chemical equation • When a chemical reaction occurs, it can be described by an equation. • This shows the chemicals that react (reactants) on the left-hand side, and the chemicals that they produce (products) on the righthand side. Reaction conditions Reactants Products Reaction between hydrogen gas ...
Chapter 4: Experimental Techniques
... applications of some of these methods are detailed. We focus on the techniques used to determine the identity and spectroscopic properties of a compound, and will not be concerned with determining the amounts of trace elements or compounds in, for example, water and air samples.† Analytical methods ...
... applications of some of these methods are detailed. We focus on the techniques used to determine the identity and spectroscopic properties of a compound, and will not be concerned with determining the amounts of trace elements or compounds in, for example, water and air samples.† Analytical methods ...
The Mole - Humble ISD
... the moles of one chemical from the given amount of a different chemical Example: How many moles of chlorine are needed to react with 5 moles of sodium (without any sodium left over)? 2 Na + Cl2 2 NaCl 5 moles Na 1 mol Cl2 2 mol Na ...
... the moles of one chemical from the given amount of a different chemical Example: How many moles of chlorine are needed to react with 5 moles of sodium (without any sodium left over)? 2 Na + Cl2 2 NaCl 5 moles Na 1 mol Cl2 2 mol Na ...
PHYSICAL SETTING CHEMISTRY
... (1) higher boiling point and a higher freezing point (2) higher boiling point and a lower freezing point (3) lower boiling point and a higher freezing point (4) lower boiling point and a lower freezing point ...
... (1) higher boiling point and a higher freezing point (2) higher boiling point and a lower freezing point (3) lower boiling point and a higher freezing point (4) lower boiling point and a lower freezing point ...
Studies on the Physiological Significance of the Lack
... reached 0.48 mg dry wt/ml (about 16 h). The cells were harvested by centrifugation (10000 g; 20 min, 4 "C) and washed twice in 10 ml of a 60 mM solution of the homologous non-radioactive compound and once in distilled water. The pellet was resuspended in distilled water (I ml) and transferred to gla ...
... reached 0.48 mg dry wt/ml (about 16 h). The cells were harvested by centrifugation (10000 g; 20 min, 4 "C) and washed twice in 10 ml of a 60 mM solution of the homologous non-radioactive compound and once in distilled water. The pellet was resuspended in distilled water (I ml) and transferred to gla ...
The Complete Notes - Joliet Junior College
... Physical and Chemical Properties – what’s the difference? Analogy: We all posses ‘as is’ physical properties, or characteristics, that define us. For example, Dr. Mills is 5’11” and has green eyes. As with people, each chemical also possesses a unique set of ‘as is’ physical properties that define ...
... Physical and Chemical Properties – what’s the difference? Analogy: We all posses ‘as is’ physical properties, or characteristics, that define us. For example, Dr. Mills is 5’11” and has green eyes. As with people, each chemical also possesses a unique set of ‘as is’ physical properties that define ...
Assigning and Using Oxidation Numbers in Biochemistry Lecture
... most important. These processes can be analyzed quantitatively by assigning redox numbers to these heteroatoms using the rules given above. The reduction of a disulfide bond to two thiol groups is a 2-electron process. An example of this is the reduction of glutathione disulfide (GCH2SSCH2G) to two ...
... most important. These processes can be analyzed quantitatively by assigning redox numbers to these heteroatoms using the rules given above. The reduction of a disulfide bond to two thiol groups is a 2-electron process. An example of this is the reduction of glutathione disulfide (GCH2SSCH2G) to two ...
Class 11 Class 12 The p- Block Element • Group13 (B to Tl
... 1 Al, Ga, In and Tl exhibit a well-defined aqueous chemistry in their tripositive states. Species like [M(OH)4 ] -, [M(H 2 O)2(OH)4 ] -, [M(OH2)6]3+ for M = Al, Ga, In, exist in aqueous solution. 2. Al, Ga. In and T1 ions exist as octahedral aqua ions, [M(OH2)6] 3 + in aqueous solution and many s ...
... 1 Al, Ga, In and Tl exhibit a well-defined aqueous chemistry in their tripositive states. Species like [M(OH)4 ] -, [M(H 2 O)2(OH)4 ] -, [M(OH2)6]3+ for M = Al, Ga, In, exist in aqueous solution. 2. Al, Ga. In and T1 ions exist as octahedral aqua ions, [M(OH2)6] 3 + in aqueous solution and many s ...
Flux Balance Analysis of Photoautotrophic
... System-wide metabolic flux characterization is recognized as an important part of metabolic engineering not only in microbial systems but also in plants (2). One approach toward a quantitative understanding of the interactions between light and central metabolism is through kinetic modeling (3, 4 an ...
... System-wide metabolic flux characterization is recognized as an important part of metabolic engineering not only in microbial systems but also in plants (2). One approach toward a quantitative understanding of the interactions between light and central metabolism is through kinetic modeling (3, 4 an ...
Modular Architecture of Metabolic Pathways Revealed by
... The RDM notation for the pair of reactants A and B is as follows: ...
... The RDM notation for the pair of reactants A and B is as follows: ...
Subwavelength transportation of light with atomic resonances
... solutions to this equation provide for truly one-dimensional “photonic band” states, examples of which have been discussed previously [30]. This provides for the waveguide modes. A. Linear chain ...
... solutions to this equation provide for truly one-dimensional “photonic band” states, examples of which have been discussed previously [30]. This provides for the waveguide modes. A. Linear chain ...
введение в общую introductio to the general ch ведение в общую
... Chemical properties of a substance are described as its abilities to form other substances in different conditions. In physical processes a substance changes at least one of its conditions: its volume, its shape, its position in the space, etc., while new substances are not formed. Phase transitions ...
... Chemical properties of a substance are described as its abilities to form other substances in different conditions. In physical processes a substance changes at least one of its conditions: its volume, its shape, its position in the space, etc., while new substances are not formed. Phase transitions ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.