Stable Isotope and Metabolomics Core Facility
... test hypotheses about the metabolic consequences of various changes in gene expression and protein function, in order to guide further integrative systems biology analyses of the underlying mechanisms in diabetes, insulin resistance, obesity, and diabetic complications. The Core objectives includes ...
... test hypotheses about the metabolic consequences of various changes in gene expression and protein function, in order to guide further integrative systems biology analyses of the underlying mechanisms in diabetes, insulin resistance, obesity, and diabetic complications. The Core objectives includes ...
Intro to Atoms Clicker Questions 1. "atomos" means? 2. Atoms of one
... 2. Atoms of one kind of element _______ be changed into a different element with ordinary chemical means. (can, can’t) 3. Every compound is composed of atoms of different elements combined how? 4. In Thompson's model of the atom, the negatively charged electrons were located how in the atom? 5. In R ...
... 2. Atoms of one kind of element _______ be changed into a different element with ordinary chemical means. (can, can’t) 3. Every compound is composed of atoms of different elements combined how? 4. In Thompson's model of the atom, the negatively charged electrons were located how in the atom? 5. In R ...
Radioisotopes
... found in nature that have been created artificially, more than 3100 nuclides are ...
... found in nature that have been created artificially, more than 3100 nuclides are ...
Chapter 19 Radioactive Material An Isotope is an element with a
... Usually, 1 isotope will go through many different decays in order to produce a stable isotope. This is called a decay series. Ex: U-‐235 (decay series) Pb-‐207 ...
... Usually, 1 isotope will go through many different decays in order to produce a stable isotope. This is called a decay series. Ex: U-‐235 (decay series) Pb-‐207 ...
atoms - Groupfusion.net
... Normally, this is the same for every atom of an element, therefore number of protons identifies the element. In a “normal”, neutral atom, number of protons = number of electrons Mass Number = the number of protons + number of neutrons = A Number of neutrons in an atom of an element can vary. These a ...
... Normally, this is the same for every atom of an element, therefore number of protons identifies the element. In a “normal”, neutral atom, number of protons = number of electrons Mass Number = the number of protons + number of neutrons = A Number of neutrons in an atom of an element can vary. These a ...
Isotopes and Ions - Wando High School
... the same atomic number, but different mass numbers (and atomic masses) CARBON (above right) 1. What is the mass number to the left? 2. What is the mass number to the right? 3. What is the atomic number to the left? 4. What is the atomic number to the right? ...
... the same atomic number, but different mass numbers (and atomic masses) CARBON (above right) 1. What is the mass number to the left? 2. What is the mass number to the right? 3. What is the atomic number to the left? 4. What is the atomic number to the right? ...
word - jpsaos
... the experiment. These questions will be due one week after performing the lab in class (your next lab class). In this lab, you will be given a bottle of beans. The beans will represent atoms. In each bottle there are three different types of beans. Each type will represent an isotope. Beanium is the ...
... the experiment. These questions will be due one week after performing the lab in class (your next lab class). In this lab, you will be given a bottle of beans. The beans will represent atoms. In each bottle there are three different types of beans. Each type will represent an isotope. Beanium is the ...
Polonium isotopes in industry Po is used in static eliminator to
... half-life (radioactive) – the time interval that it takes for the total number of atoms of any radioactive isotope to decay and leave only one-half of the original number of atoms. [return] ionizing – pertaining to the process by which an atom, molecule, or substance acquires a negative or positive ...
... half-life (radioactive) – the time interval that it takes for the total number of atoms of any radioactive isotope to decay and leave only one-half of the original number of atoms. [return] ionizing – pertaining to the process by which an atom, molecule, or substance acquires a negative or positive ...
Atomic Structure - hrsbstaff.ednet.ns.ca
... 2. Within atoms of the same element, the number of ________________will vary from one atom to the next. These various form of the element are called ___________________. 3. All the isotopes of a particular element have the same ___________________ but they have different ____________________________ ...
... 2. Within atoms of the same element, the number of ________________will vary from one atom to the next. These various form of the element are called ___________________. 3. All the isotopes of a particular element have the same ___________________ but they have different ____________________________ ...
Unit 2 Notes - School City of Hobart
... Nuclear reactions involve changes in atomic nuclei to generate energy. Nuclear Chemistry is the study of those reactions, with an emphasis on their uses in chemistry and their effects on biological systems 21.1 Radioactivity • Nucleon is simply another name for particles in the nucleus (proton/neutr ...
... Nuclear reactions involve changes in atomic nuclei to generate energy. Nuclear Chemistry is the study of those reactions, with an emphasis on their uses in chemistry and their effects on biological systems 21.1 Radioactivity • Nucleon is simply another name for particles in the nucleus (proton/neutr ...
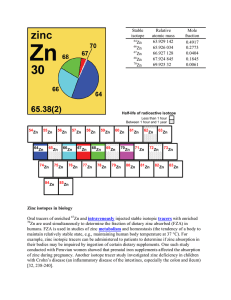
Zinc isotopes in biology Oral tracers of enriched Zn and
... anthropogenic – resulting from human activity. [return] atomic number (Z) – The number of protons in the nucleus of an atom. atomic weight (relative mean atomic mass) – the sum of the products of the relative atomic mass and the mole fraction of each stable and long-lived radioactive isotope of that ...
... anthropogenic – resulting from human activity. [return] atomic number (Z) – The number of protons in the nucleus of an atom. atomic weight (relative mean atomic mass) – the sum of the products of the relative atomic mass and the mole fraction of each stable and long-lived radioactive isotope of that ...
2 - My George School
... • Alpha particles shot at a thin gold foil • Some are _______, some go ________, some __________! – Proves atoms have positively charged nucleus – Disproves Lord Kelvin’s __________________ ...
... • Alpha particles shot at a thin gold foil • Some are _______, some go ________, some __________! – Proves atoms have positively charged nucleus – Disproves Lord Kelvin’s __________________ ...
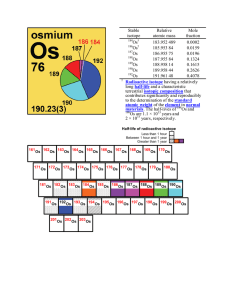
Rhenium isotopes in geochronology Stable isotope Relative atomic
... for industry or science; the material is not itself studied for some extraordinary anomaly and its mole fractions (isotopic abundances) have not been modified significantly in a geologically brief period [4]. [return] palliative – providing relief from the symptoms of an illness without treating its ...
... for industry or science; the material is not itself studied for some extraordinary anomaly and its mole fractions (isotopic abundances) have not been modified significantly in a geologically brief period [4]. [return] palliative – providing relief from the symptoms of an illness without treating its ...
File
... but how can my love not be revealed? My mass won’t be complete until you’re mine, so give me this chance, and show me a sign. Some day we’ll meet and I'll see your eyes, and we will be together and neutralize... so until that day, farewell my opposite attract, ...
... but how can my love not be revealed? My mass won’t be complete until you’re mine, so give me this chance, and show me a sign. Some day we’ll meet and I'll see your eyes, and we will be together and neutralize... so until that day, farewell my opposite attract, ...
Build an Atom
... Introduction: Breath in…Breath out. Again! When you inhale air, you are not just inhaling a mixture of oxygen, nitrogen, and trace gasses, but a mixture of different oxygen atoms and different nitrogen atoms. It turns out that all oxygen atoms have the same number of protons, but some may have diffe ...
... Introduction: Breath in…Breath out. Again! When you inhale air, you are not just inhaling a mixture of oxygen, nitrogen, and trace gasses, but a mixture of different oxygen atoms and different nitrogen atoms. It turns out that all oxygen atoms have the same number of protons, but some may have diffe ...
Chapter 14 Review
... 16. The half-life of Nobelium is 3 minutes. How much Nobelium will there be in 12 minutes if the original sample was 48 grams? What has happened to the other part of the sample? ...
... 16. The half-life of Nobelium is 3 minutes. How much Nobelium will there be in 12 minutes if the original sample was 48 grams? What has happened to the other part of the sample? ...
2.1 The Nature of Matter - Sonoma Valley High School
... Some elements have isotopes, with different #s of neutrons and different mass. All isotopes of an element have the same chemical properties b/c their electrons are the same. ...
... Some elements have isotopes, with different #s of neutrons and different mass. All isotopes of an element have the same chemical properties b/c their electrons are the same. ...
Stable isotope Relative atomic mass Mole fraction Os 183.952 489
... number of protons in the nucleus of an atom is the atomic number. radioactive decay – the process by which unstable (or radioactive) isotopes lose energy by emitting alpha particles (helium nuclei), beta particles (positive or negative electrons), gamma radiation, neutrons or protons to reach a fina ...
... number of protons in the nucleus of an atom is the atomic number. radioactive decay – the process by which unstable (or radioactive) isotopes lose energy by emitting alpha particles (helium nuclei), beta particles (positive or negative electrons), gamma radiation, neutrons or protons to reach a fina ...
Name
... 1. The minimum mass of fissionable material in a reactor or nuclear bomb that will sustain a chain reaction. 5. A self-sustaining reaction in which the products of one reaction event stimulate further reaction events 8. The tendency of some elements, such as uranium, to emit radiation as a result of ...
... 1. The minimum mass of fissionable material in a reactor or nuclear bomb that will sustain a chain reaction. 5. A self-sustaining reaction in which the products of one reaction event stimulate further reaction events 8. The tendency of some elements, such as uranium, to emit radiation as a result of ...
The discovery of the natural radioactive decay of uranium in 1896 by
... different atomic weights owing to variations in the number of neutrons. Atoms of the same element with differing atomic weights are called isotopes. Radioactive decay is a spontaneous process in which an isotope (the parent) loses particles from its nucleus to form an isotope of a new element (the d ...
... different atomic weights owing to variations in the number of neutrons. Atoms of the same element with differing atomic weights are called isotopes. Radioactive decay is a spontaneous process in which an isotope (the parent) loses particles from its nucleus to form an isotope of a new element (the d ...
Worksheet
... 15. __________ He used the term “atomos” to describe an indivisible part at the base of all matter. 16. __________ He is the Father of the Atomic Theory. 17. __________ He designed a mathematical equation for the model of the atom. 18. __________ He determined the charge of the electron. 19. _______ ...
... 15. __________ He used the term “atomos” to describe an indivisible part at the base of all matter. 16. __________ He is the Father of the Atomic Theory. 17. __________ He designed a mathematical equation for the model of the atom. 18. __________ He determined the charge of the electron. 19. _______ ...
Principles of Matter and Energy
... By determining ratios of the different isotopes of carbon and other elements in samples of biological origin and in rocks, scientists are able to determine with certainty when these materials formed. ...
... By determining ratios of the different isotopes of carbon and other elements in samples of biological origin and in rocks, scientists are able to determine with certainty when these materials formed. ...
Isotope Notes
... b. EQ #13: How can you calculate the number of protons, neutrons, and electrons for an isotope of an element? 2. Isotopes and Mass a. ISOTOPES are atoms of the ______________________ that have the same number of protons but different numbers of ___________________ i. They have different masses. b. D ...
... b. EQ #13: How can you calculate the number of protons, neutrons, and electrons for an isotope of an element? 2. Isotopes and Mass a. ISOTOPES are atoms of the ______________________ that have the same number of protons but different numbers of ___________________ i. They have different masses. b. D ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.