Ch-03 Notes
... identical in size, mass and other properties different from those of the other elements 3) Atoms cannot be subdivided, created or destroyed 4) Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative number and types of atoms 5) I ...
... identical in size, mass and other properties different from those of the other elements 3) Atoms cannot be subdivided, created or destroyed 4) Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative number and types of atoms 5) I ...
Unit 2 Spiraling
... 7. What parts of Dalton’s atomic theory no longer agree with the current picture of the atom? 8.. Dalton’s atomic theory was not correct in every detail. Should this be taken as criticism of Dalton as a scientist? Explain. 9. What was inadequate about Rutherford’s model of the atom? 10. What did Boh ...
... 7. What parts of Dalton’s atomic theory no longer agree with the current picture of the atom? 8.. Dalton’s atomic theory was not correct in every detail. Should this be taken as criticism of Dalton as a scientist? Explain. 9. What was inadequate about Rutherford’s model of the atom? 10. What did Boh ...
Laws
... • During a chemical reaction, a group combines 5.00 grams of sodium and 7.72 grams of chlorine. The result of the reaction was 12.72 grams of sodium chloride. Which law does this support? ...
... • During a chemical reaction, a group combines 5.00 grams of sodium and 7.72 grams of chlorine. The result of the reaction was 12.72 grams of sodium chloride. Which law does this support? ...
Enrichment of Germanium-73 with the Magnetic Isotope Effect
... Here, &*/A0 and A * / A represent the magnetic isotope abundance obtained before and after photolysis. The isotope selection coefficient,a,is a very important parameter governing the isotope enrichment of starting compound. The isotope enrichment, E, can be estimated from the 6(=Ge) value. In Figure ...
... Here, &*/A0 and A * / A represent the magnetic isotope abundance obtained before and after photolysis. The isotope selection coefficient,a,is a very important parameter governing the isotope enrichment of starting compound. The isotope enrichment, E, can be estimated from the 6(=Ge) value. In Figure ...
Atomic Structure
... Isotopes are atoms of the same element having different masses, due to varying numbers of neutrons. ...
... Isotopes are atoms of the same element having different masses, due to varying numbers of neutrons. ...
CHEMISTRY 1 FINAL EXAM REVIEW
... 3.) List each as being a physical change or a chemical change: butter melting, butter burning, sugar dissolving in water, a sandwich getting digested. 4.) List each as being a physical or a chemical property: copper sulfate is blue, iron is a solid, water o ...
... 3.) List each as being a physical change or a chemical change: butter melting, butter burning, sugar dissolving in water, a sandwich getting digested. 4.) List each as being a physical or a chemical property: copper sulfate is blue, iron is a solid, water o ...
Unit 3 Review Sheet – Biochemistry
... What are the characteristics of water that make it important to life? Polar, high heat capacity, resists temperature change, abililty to bond and attract other molecules (cohesion and adhesion), ice is less dense than liquid water, universal solvent, most abundant compound in living things What does ...
... What are the characteristics of water that make it important to life? Polar, high heat capacity, resists temperature change, abililty to bond and attract other molecules (cohesion and adhesion), ice is less dense than liquid water, universal solvent, most abundant compound in living things What does ...
Problem Set
... Example 4-9. Determine the wavelength, in m, of an electron, with mass 9.11 x 10-31 kg, having a velocity of 5.65 x 107 m/s. – Remember Planck’s constant is 6.626 x 10-34 Js which is also equal to 6.626 x 10-34 kg m2/s. ...
... Example 4-9. Determine the wavelength, in m, of an electron, with mass 9.11 x 10-31 kg, having a velocity of 5.65 x 107 m/s. – Remember Planck’s constant is 6.626 x 10-34 Js which is also equal to 6.626 x 10-34 kg m2/s. ...
Chem 1721 Brief Notes: Chapter 20 Chapter 20: Nuclear Chemistry
... atomic number = # protons in the nucleus; this does not change for atoms of a specific element i.e. every atom of calcium has 20 protons in the nucleus if the number of protons changes the identity of the element changes mass number = # protons + # neutrons; mass number can change because the number ...
... atomic number = # protons in the nucleus; this does not change for atoms of a specific element i.e. every atom of calcium has 20 protons in the nucleus if the number of protons changes the identity of the element changes mass number = # protons + # neutrons; mass number can change because the number ...
Unit 1: Stoichiometry
... There are two naturally occurring isotopes of chlorine: chlorine‐35 and chlorine‐37. The atomic mass of this element is a combination of the two isotopes. The relative abundance of chlorine atoms in nature is 75% chlorine‐35 and 25% chlorine‐37. Average atomic mass is the weighted average of the ato ...
... There are two naturally occurring isotopes of chlorine: chlorine‐35 and chlorine‐37. The atomic mass of this element is a combination of the two isotopes. The relative abundance of chlorine atoms in nature is 75% chlorine‐35 and 25% chlorine‐37. Average atomic mass is the weighted average of the ato ...
Some isotopes - Red Hook Central School District
... same number of PROTONS in its nucleus • For example, for Lithium to be Lithium, it must have 3 protons. An element with only two protons would be He, with 4 protons Be ...
... same number of PROTONS in its nucleus • For example, for Lithium to be Lithium, it must have 3 protons. An element with only two protons would be He, with 4 protons Be ...
Carbon Compounds in Cells
... – Examples: Starch: Plant storage for energy; unbranched coil chain and easily hydrolyzed – Cellulose: Found in plants for structure ...
... – Examples: Starch: Plant storage for energy; unbranched coil chain and easily hydrolyzed – Cellulose: Found in plants for structure ...
Original
... acids bond to form a dipeptide. In this condensation reaction, the two amino acids form a covalent bond (peptide bond) and release a water molecule. ...
... acids bond to form a dipeptide. In this condensation reaction, the two amino acids form a covalent bond (peptide bond) and release a water molecule. ...
Matter and Energy
... Properties of Matter Practice 1. Describe each of the following properties as physical or chemical: a. neon is a color gas at room temperature b. apple slices turn brown when exposed to air c. phosphorus will ignite when exposed to air d. at room temperature, mercury is a liquid e. propane gas is c ...
... Properties of Matter Practice 1. Describe each of the following properties as physical or chemical: a. neon is a color gas at room temperature b. apple slices turn brown when exposed to air c. phosphorus will ignite when exposed to air d. at room temperature, mercury is a liquid e. propane gas is c ...
Chapter 3 - cloudfront.net
... 1) All ________ is composed of tiny indivisible particles called atoms 2) Atoms of the same __________ are identical – size, mass, etc… Atoms of different elements are different. 3) Atoms of different elements combine in simple whole-number ratios to form _________________ 4) In chemical reactions, ...
... 1) All ________ is composed of tiny indivisible particles called atoms 2) Atoms of the same __________ are identical – size, mass, etc… Atoms of different elements are different. 3) Atoms of different elements combine in simple whole-number ratios to form _________________ 4) In chemical reactions, ...
Atoms - ChemConnections
... The understanding that matter is composed of different elements evolved over thousands of years. Substances such as gold and silver were known in ancient times, but they were not understood to be elements. The alchemists, who did not understand elements or atoms, tried to change substances into gold ...
... The understanding that matter is composed of different elements evolved over thousands of years. Substances such as gold and silver were known in ancient times, but they were not understood to be elements. The alchemists, who did not understand elements or atoms, tried to change substances into gold ...
1. Sucrose is a disaccharide. It is formed from two
... The enzyme sucrase catalyses the breakdown of sucrose into monosaccharides. What type of reaction is this breakdown? ...
... The enzyme sucrase catalyses the breakdown of sucrose into monosaccharides. What type of reaction is this breakdown? ...
Chapter 2 (Hill/Petrucci/McCreary/Perry This chapter deals with
... 1. all matter is composed of small, invisible particles called atoms 2. in chemical reactions, atoms are neither created nor destroyed 3. atoms of each element have unique properties - all atoms of a given atom are identical and have identical masses and other properties 4. chemical reactions involv ...
... 1. all matter is composed of small, invisible particles called atoms 2. in chemical reactions, atoms are neither created nor destroyed 3. atoms of each element have unique properties - all atoms of a given atom are identical and have identical masses and other properties 4. chemical reactions involv ...
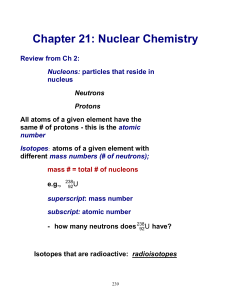
Chapter 21: Nuclear Chemistry
... radiation This can transform the unstable nucleus into a stable one - the emitted radiation carries off extra energy e.g., decay of uranium-238 by spontaneous emission of particles: ...
... radiation This can transform the unstable nucleus into a stable one - the emitted radiation carries off extra energy e.g., decay of uranium-238 by spontaneous emission of particles: ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.