Document
... The energy that flows into or out of a system because of a difference in temperature between the thermodynamic system and its surroundings. Heat flows spontaneously from a region of higher temperature to a region of lower temperature. • q is defined as positive if heat is absorbed by the system (hea ...
... The energy that flows into or out of a system because of a difference in temperature between the thermodynamic system and its surroundings. Heat flows spontaneously from a region of higher temperature to a region of lower temperature. • q is defined as positive if heat is absorbed by the system (hea ...
Single-Replacement Reactions
... We need one more oxygen in the products. Can’t change the formula, because it describes what it is (carbon monoxide in this example) ...
... We need one more oxygen in the products. Can’t change the formula, because it describes what it is (carbon monoxide in this example) ...
File
... compound given data such as the masses of CO2 and H2O formed in a combustion reaction. Use mass, mole, stoichiometry, and/or combustion information to calculate the empirical formula and molecular formula of an unknown substance. ...
... compound given data such as the masses of CO2 and H2O formed in a combustion reaction. Use mass, mole, stoichiometry, and/or combustion information to calculate the empirical formula and molecular formula of an unknown substance. ...
C2 Knowledge PowerPoint
... Many chemical processes use catalysts to increase rate of production of products Catalysts help to lower the temperature and pressure needed = less energy needed = saves money ...
... Many chemical processes use catalysts to increase rate of production of products Catalysts help to lower the temperature and pressure needed = less energy needed = saves money ...
Document
... • During a chemical reaction there is usually a transfer of energy between the reactant and the surroundings. ...
... • During a chemical reaction there is usually a transfer of energy between the reactant and the surroundings. ...
284
... e. 0.835 mol of iron(II) sulfide f. 4.01 mol of potassium hydroxide g. 0.0219 mol of. hydrogen gas 23. For each of the following unbalanced equations, indicate how many moles of each product could be produced by complete reaction of 1.00 g of the reactant indicated in boldface. a. LiOH(s) + CO2(g) ...
... e. 0.835 mol of iron(II) sulfide f. 4.01 mol of potassium hydroxide g. 0.0219 mol of. hydrogen gas 23. For each of the following unbalanced equations, indicate how many moles of each product could be produced by complete reaction of 1.00 g of the reactant indicated in boldface. a. LiOH(s) + CO2(g) ...
2005 - NESACS
... II. A central atom of a molecule has a lone pairs of electrons on it. III. The molecule's electronic geometry and molecular geometry are the same. (A) (B) (C) (D) ...
... II. A central atom of a molecule has a lone pairs of electrons on it. III. The molecule's electronic geometry and molecular geometry are the same. (A) (B) (C) (D) ...
- Jersey College For Girls
... (ii) Isotopes are atoms with the same number of .................................................................... but different numbers of .................................................................... in the nucleus. ...
... (ii) Isotopes are atoms with the same number of .................................................................... but different numbers of .................................................................... in the nucleus. ...
2010 - SAASTA
... conditions (e.g., temperature, pressure or concentration) of an equilibrium system are changed, the reaction which tends to cancel the effect of the changes will be favoured. In the above example, four moles of gas are converted into 2 moles of gas in the forward reaction and thus when the pressure ...
... conditions (e.g., temperature, pressure or concentration) of an equilibrium system are changed, the reaction which tends to cancel the effect of the changes will be favoured. In the above example, four moles of gas are converted into 2 moles of gas in the forward reaction and thus when the pressure ...
CHEMISTRY I Final..#1..rev 4KEY
... 38. The boiling point of HBr is lower than that of HF because: a. HBr is heavier than HF and therefore it requires less energy to vaporize. b. HBr has dipole-dipole attractions which are weaker than the hydrogen bonding found in HF. c. The dispersion forces are weaker in HBr than in HF. d. All of th ...
... 38. The boiling point of HBr is lower than that of HF because: a. HBr is heavier than HF and therefore it requires less energy to vaporize. b. HBr has dipole-dipole attractions which are weaker than the hydrogen bonding found in HF. c. The dispersion forces are weaker in HBr than in HF. d. All of th ...
Chemical Equations and Reactions
... A formula equation is an equation in which the reactants and products are represented by symbols and formulas. It has only qualitative meaning, until the equation is balanced are given. Provide valuable information such as the number of moles or atoms of the elements or formulas contained in the equ ...
... A formula equation is an equation in which the reactants and products are represented by symbols and formulas. It has only qualitative meaning, until the equation is balanced are given. Provide valuable information such as the number of moles or atoms of the elements or formulas contained in the equ ...
Question paper - Edexcel
... Use this space for any rough working. Anything you write in this space will gain no credit. ...
... Use this space for any rough working. Anything you write in this space will gain no credit. ...
Activity Series Unit
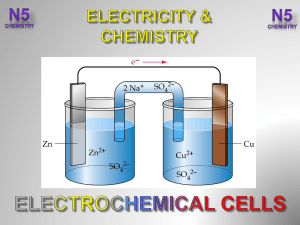
... 29. Generally speaking, what happens to these species in these reactions? They are gaining electrons from the metals. 30. When looking at electrons, what can be said about the term, reduction? Reduction means the gain of electrons. 31. In these reactions what species is causing reduction? What spec ...
... 29. Generally speaking, what happens to these species in these reactions? They are gaining electrons from the metals. 30. When looking at electrons, what can be said about the term, reduction? Reduction means the gain of electrons. 31. In these reactions what species is causing reduction? What spec ...
Chem Final Study Guide Energy How much heat energy must be
... 68) What is the pH of a solution that has an OH- ion concentration of 1× 10-3 mole per liter? Is it an acid or base? a) pH of 11, base 69) What is the H+ of an HCl solution if the pH is measured to be 4? a) 1 x 10-4 70) What is a characteristic of a strong acid? a) Good electrolyte 71) What is the d ...
... 68) What is the pH of a solution that has an OH- ion concentration of 1× 10-3 mole per liter? Is it an acid or base? a) pH of 11, base 69) What is the H+ of an HCl solution if the pH is measured to be 4? a) 1 x 10-4 70) What is a characteristic of a strong acid? a) Good electrolyte 71) What is the d ...
Chapter 8 Thermochemistry: Chemical Energy
... Standard Heats of Formation Standard Heat of Formation (DHof ): The enthalpy change for the formation of 1 mol of a substance in its standard state from its constituent elements in their ...
... Standard Heats of Formation Standard Heat of Formation (DHof ): The enthalpy change for the formation of 1 mol of a substance in its standard state from its constituent elements in their ...
Ch 17 practice assessment w
... package and sealing. Some perishable items can be sensitive to changes in temperature and humidity. If they are to stay fresh for the longest possible time, they need to be kept in a controlled environment. But, how can this be accomplished if they are traveling in a truck through different weather ...
... package and sealing. Some perishable items can be sensitive to changes in temperature and humidity. If they are to stay fresh for the longest possible time, they need to be kept in a controlled environment. But, how can this be accomplished if they are traveling in a truck through different weather ...
Class Notes
... of hydrogen peroxide at home turning into water and oxygen gas? No, not very quickly at least. There are a lot of factors that can affect whether a reaction will occur spontaneously or not. These include: bond strength, temperature, motion of compounds, and concentration of the reactants. Some react ...
... of hydrogen peroxide at home turning into water and oxygen gas? No, not very quickly at least. There are a lot of factors that can affect whether a reaction will occur spontaneously or not. These include: bond strength, temperature, motion of compounds, and concentration of the reactants. Some react ...
Type of Chemical Reactions Lab
... The manganese dioxide in Rxn 4 is a catalyst – a chemical that speeds up a reaction without being used up. The catalyst should be written above the ‘yields’ arrow. It should NOT appear as a reactant for the reaction. The salt and indicator in Rxn 5 do not get included in the reaction. The salt i ...
... The manganese dioxide in Rxn 4 is a catalyst – a chemical that speeds up a reaction without being used up. The catalyst should be written above the ‘yields’ arrow. It should NOT appear as a reactant for the reaction. The salt and indicator in Rxn 5 do not get included in the reaction. The salt i ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.