Chapter 2 1.Certain gases in the 293K and 9.97 × 104Pa when the
... pressure at this condition Solution:From the meaning of the questions to know the temperature when the bottle was promoted to 500K, its size will become the original five thirds times. Therefore, the amount of bottle gases account for only three-fifths of all the gas, the corresponding pressure 1013 ...
... pressure at this condition Solution:From the meaning of the questions to know the temperature when the bottle was promoted to 500K, its size will become the original five thirds times. Therefore, the amount of bottle gases account for only three-fifths of all the gas, the corresponding pressure 1013 ...
1 AM SYLLABUS (2015) CHEMISTRY AM 06 SYLLABUS
... Plot two variables from given data; understand that y = mx + c represents a linear relationship and be able to determine the slope and intercept of a line; draw and use the slope of a tangent to a curve as a measure of rate of change. ...
... Plot two variables from given data; understand that y = mx + c represents a linear relationship and be able to determine the slope and intercept of a line; draw and use the slope of a tangent to a curve as a measure of rate of change. ...
МЕТОДИЧЕСКИЕ УКАЗАНИЯ СТУДЕНТАМ
... • All the experiments with foul-smelling as well as poisonous substances (aniline, bromine) are done in the exhaust-hood. • When working with a drain tube, you can take away the burner from under the test tube with the regent mixture only when the end of the pipe is removed from the liquid. Other wi ...
... • All the experiments with foul-smelling as well as poisonous substances (aniline, bromine) are done in the exhaust-hood. • When working with a drain tube, you can take away the burner from under the test tube with the regent mixture only when the end of the pipe is removed from the liquid. Other wi ...
... Chemical warfare and pollution of the environment as well as drug abuse, adulteration of food and similar examples of misuse or improper use or disposal of chemicals often cast shadows on this scientific enterprise and generate mistrust of chemicals by the general public. The main purpose of this sy ...
Electrochemical Preparation of Strong Bases Henning Lund
... with intermediate adsorbed hydrogen. Bases from acids with pKA-values up to 20 have been prepared by this method [2]. In organic chemistry even stronger bases are sometimes required for synthesis, and in such cases the anion of DMSO (“dimsyl”) may be employed. This reagent can be prepared by reactin ...
... with intermediate adsorbed hydrogen. Bases from acids with pKA-values up to 20 have been prepared by this method [2]. In organic chemistry even stronger bases are sometimes required for synthesis, and in such cases the anion of DMSO (“dimsyl”) may be employed. This reagent can be prepared by reactin ...
Exam
... 32) Is a solution of sodium phosphate in water acidic or basic? 33) In a titration experiment, a student used 24.13 mL of 0.111 M sodium hydroxide to neutralize 20.00 mL of a hydrochloric acid solution. What was the molarity of the acid solution? 34) A student had 2.0 L of a sodium hydroxide soluti ...
... 32) Is a solution of sodium phosphate in water acidic or basic? 33) In a titration experiment, a student used 24.13 mL of 0.111 M sodium hydroxide to neutralize 20.00 mL of a hydrochloric acid solution. What was the molarity of the acid solution? 34) A student had 2.0 L of a sodium hydroxide soluti ...
The Chemistry Handbook - A2
... It is essential that you learn all the definitions you come across (and there really is NO substitute to just memorizing them). The text books you are provided with have a glossary of definitions at the back of the book. Use the table below to record any that you want to have quick reference to. It ...
... It is essential that you learn all the definitions you come across (and there really is NO substitute to just memorizing them). The text books you are provided with have a glossary of definitions at the back of the book. Use the table below to record any that you want to have quick reference to. It ...
The impact of pore water chemistry on carbonate surface charge
... is the partial pressure of a gas phase. Specifying the total solution concentration of a basis species (and all its complexes) is also a valid constraint that can be substituted for a buffer mineral. This method of calculating solution composition has been developed and extensively tested over many ...
... is the partial pressure of a gas phase. Specifying the total solution concentration of a basis species (and all its complexes) is also a valid constraint that can be substituted for a buffer mineral. This method of calculating solution composition has been developed and extensively tested over many ...
C6 Revision Guide - West Derby School
... When a soap flake is shaken in a water sample, calcium ions in the water (from the calcium hydrogencarbonate) react with the soap to form a nasty scum. As you add more flakes, over time and after you’ve continually shaken the mixture, the soap reacts with ALL of the calcium ions. After this point, a ...
... When a soap flake is shaken in a water sample, calcium ions in the water (from the calcium hydrogencarbonate) react with the soap to form a nasty scum. As you add more flakes, over time and after you’ve continually shaken the mixture, the soap reacts with ALL of the calcium ions. After this point, a ...
Strecker Degradation Products of Aspartic and Glutamic Acids and
... Aspartic and glutamic acids, asparagine and glutamine were oxidised with either potassium peroxodisulphate or glyoxal. Nonvolatile products were derivatised and analysed by GC/FID and GC/MS. Volatile reaction products were isolated and analysed by the same methods. It was found that the degradation ...
... Aspartic and glutamic acids, asparagine and glutamine were oxidised with either potassium peroxodisulphate or glyoxal. Nonvolatile products were derivatised and analysed by GC/FID and GC/MS. Volatile reaction products were isolated and analysed by the same methods. It was found that the degradation ...
Mineralization of Drugs in Aqueous Medium by Advanced Oxidation
... environments following a natural cycle. The increasing lack and contamination of this natural resource in many places of the world is alarming and demands urgent solutions. Recently, there is great interest in the environmental relevance of contamination of natural waters by pharmaceutical drugs and ...
... environments following a natural cycle. The increasing lack and contamination of this natural resource in many places of the world is alarming and demands urgent solutions. Recently, there is great interest in the environmental relevance of contamination of natural waters by pharmaceutical drugs and ...
chap15pptlecture_chapte.ppt [Read-Only]
... If a reaction can be expressed as the sum of two or more reactions, the equilibrium constant for the overall reaction is given by the product of the equilibrium constants of the individual reactions. ...
... If a reaction can be expressed as the sum of two or more reactions, the equilibrium constant for the overall reaction is given by the product of the equilibrium constants of the individual reactions. ...
ExamView - 2002 AP Chemistry Exam.tst
... (1) Test Questions are Copyright © 1984-2002 by College Entrance Examination Board, Princeton, NJ. All rights reserved. For face-to-face teaching purposes, classroom teachers are permitted to reproduce the questions. Web or Mass distribution prohibited. (2) AP® is a registered trademark of the Colle ...
... (1) Test Questions are Copyright © 1984-2002 by College Entrance Examination Board, Princeton, NJ. All rights reserved. For face-to-face teaching purposes, classroom teachers are permitted to reproduce the questions. Web or Mass distribution prohibited. (2) AP® is a registered trademark of the Colle ...
importance of chemistry in geothermal exploration and utilization
... The concentration of most elements in geothermal water is dependent on temperature. Mixing of geothermal water and colder water often causes deviation from equilibrium. Changes in the chemical composition of geothermal water caused by invasion of cold ground water may precede physical changes. The r ...
... The concentration of most elements in geothermal water is dependent on temperature. Mixing of geothermal water and colder water often causes deviation from equilibrium. Changes in the chemical composition of geothermal water caused by invasion of cold ground water may precede physical changes. The r ...
Now! - Soojeede.com
... Non-electrolytes have been described as solutions that do not conduct electricity and electrolytes are those that do conduct electricity. However, electrolytes do have varying degrees of strength. If a solution has a large number of ions present in it, it is called a strong electrolyte whereas an el ...
... Non-electrolytes have been described as solutions that do not conduct electricity and electrolytes are those that do conduct electricity. However, electrolytes do have varying degrees of strength. If a solution has a large number of ions present in it, it is called a strong electrolyte whereas an el ...
Word Pro
... If the final reaction product mixture from a reaction contains 2.0 mol of N2(g), 2.0 mol of H2(g) and 2.0 mol of NH3(g), how many moles of N2(g) and H2(g) were present at the start of the reaction? (i.e. before any NH3 formed)? ...
... If the final reaction product mixture from a reaction contains 2.0 mol of N2(g), 2.0 mol of H2(g) and 2.0 mol of NH3(g), how many moles of N2(g) and H2(g) were present at the start of the reaction? (i.e. before any NH3 formed)? ...
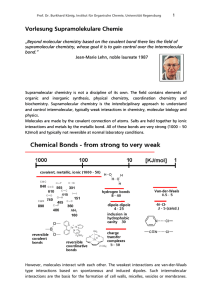
Vorlesung Supramolekulare Chemie
... π – π Stacking (0-50 KJ/mol): It is a weak electrostatic interaction, typically between aromatic rings with one binding partner more electron rich, the other more electron poor. There are two possible orientations observed in this interaction: A face to face orientation and an edge to face orientati ...
... π – π Stacking (0-50 KJ/mol): It is a weak electrostatic interaction, typically between aromatic rings with one binding partner more electron rich, the other more electron poor. There are two possible orientations observed in this interaction: A face to face orientation and an edge to face orientati ...
STOICHIOMETRY REVIEW WORKSHEET
... Part 2: Solve the following stoichiometry grams-grams problems: 1) The combustion of a sample of butane, C4H10 (lighter fluid), produced 2.46 grams of water. C4H10 + ...
... Part 2: Solve the following stoichiometry grams-grams problems: 1) The combustion of a sample of butane, C4H10 (lighter fluid), produced 2.46 grams of water. C4H10 + ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.















![chap15pptlecture_chapte.ppt [Read-Only]](http://s1.studyres.com/store/data/015369082_1-00cbf06a2d468a4ae1c963f5ca674e31-300x300.png)