Example of Lab Notebook
... were not calibrated ahead of time, the masses and volumes used throughout the experiment could be unreliable. The purity of the product was assessed by comparing the observed melting point to the theoretical range. The observed melting point of the product was 111.5–115oC which was close to the theo ...
... were not calibrated ahead of time, the masses and volumes used throughout the experiment could be unreliable. The purity of the product was assessed by comparing the observed melting point to the theoretical range. The observed melting point of the product was 111.5–115oC which was close to the theo ...
Solutions for Practice Problems
... The volume of oxygen that is required is 15.6 L. Check Your Solution The volume of oxygen gas seems reasonable given the mole ratio in the balanced chemical equation and the mass of iron that reacted. The answer correctly shows three significant digits. 36. Practice Problem (page 560) Oxygen gas and ...
... The volume of oxygen that is required is 15.6 L. Check Your Solution The volume of oxygen gas seems reasonable given the mole ratio in the balanced chemical equation and the mass of iron that reacted. The answer correctly shows three significant digits. 36. Practice Problem (page 560) Oxygen gas and ...
Chapter 17 - Cengage Learning
... Increases in the temperature and concentration of reactants bring about more collisions, and the rate of reaction increases. The collision model explains many observations about reactions. Not all molecules that collide react. Colliding molecules must have a minimum amount of energy for a reaction t ...
... Increases in the temperature and concentration of reactants bring about more collisions, and the rate of reaction increases. The collision model explains many observations about reactions. Not all molecules that collide react. Colliding molecules must have a minimum amount of energy for a reaction t ...
On the Importance of Prereactive Complexes in
... several explanations have been proposed, which are summarized in ref 2. Three of them maintain the idea of an elementary reaction but suggest a modification of the preexponential factor in the Arrhenius equation to allow for a term T-1.5. Singleton and Cvetanovic11 propose a complex mechanism and ex ...
... several explanations have been proposed, which are summarized in ref 2. Three of them maintain the idea of an elementary reaction but suggest a modification of the preexponential factor in the Arrhenius equation to allow for a term T-1.5. Singleton and Cvetanovic11 propose a complex mechanism and ex ...
Reaction Rates/Chemical Kinetics
... Sample Exercise 15.12 Calculating Equilibrium Concentrations from Initial Concentrations Solution (continued) Third, we use the stoichiometry of the reaction to determine the changes in concentration that occur as the reaction proceeds to equilibrium. The concentrations of H2 and I2 will decrease a ...
... Sample Exercise 15.12 Calculating Equilibrium Concentrations from Initial Concentrations Solution (continued) Third, we use the stoichiometry of the reaction to determine the changes in concentration that occur as the reaction proceeds to equilibrium. The concentrations of H2 and I2 will decrease a ...
Unit 4 - Calculations and Chemical Reactions
... Notice that the K+ and NO3- and ions don’t undergo chemical changes. They are in the exact same form on both sides of the equation. Ions that don’t undergo a chemical change during a chemical reaction are called spectator ions. If we omit the spectator ions, we will have the net ionic equation: Ag+( ...
... Notice that the K+ and NO3- and ions don’t undergo chemical changes. They are in the exact same form on both sides of the equation. Ions that don’t undergo a chemical change during a chemical reaction are called spectator ions. If we omit the spectator ions, we will have the net ionic equation: Ag+( ...
7.1 Describing Reactions
... Describing Ionic Compounds 1. Hydrogen chloride, or HCl, is an important industrial chemical. Write a balanced equation for the production of hydrogen chloride from hydrogen and chlorine. Answer: H2 + Cl2 2HCl ...
... Describing Ionic Compounds 1. Hydrogen chloride, or HCl, is an important industrial chemical. Write a balanced equation for the production of hydrogen chloride from hydrogen and chlorine. Answer: H2 + Cl2 2HCl ...
Name:
... According to these results, what would be the initial rate (in mol/(L·s)) if all three concentrations are: [BrO3-]=[Br-]=[H+]=0.20 mol/L? 2. Use the following diagram to answer the questions below. a) Is the reaction exothermic or endothermic? Explain. b) What letter represents the activation energy ...
... According to these results, what would be the initial rate (in mol/(L·s)) if all three concentrations are: [BrO3-]=[Br-]=[H+]=0.20 mol/L? 2. Use the following diagram to answer the questions below. a) Is the reaction exothermic or endothermic? Explain. b) What letter represents the activation energy ...
7.1 Describing Reactions
... Describing Ionic Compounds 1. Hydrogen chloride, or HCl, is an important industrial chemical. Write a balanced equation for the production of hydrogen chloride from hydrogen and chlorine. Answer: H2 + Cl2 2HCl ...
... Describing Ionic Compounds 1. Hydrogen chloride, or HCl, is an important industrial chemical. Write a balanced equation for the production of hydrogen chloride from hydrogen and chlorine. Answer: H2 + Cl2 2HCl ...
California Standards Practice - Student Edition
... solutions. As a basis for understanding this concept: a. Students know the observable properties of acids, bases, and salt solutions. b. Students know acids are hydrogen-ion-donating and bases are hydrogenion-accepting substances. c. Students know strong acids and bases fully dissociate and weak aci ...
... solutions. As a basis for understanding this concept: a. Students know the observable properties of acids, bases, and salt solutions. b. Students know acids are hydrogen-ion-donating and bases are hydrogenion-accepting substances. c. Students know strong acids and bases fully dissociate and weak aci ...
JEE Main 2014 1. The current voltage relation of diode is given by I
... where c is the critical angle and is the refractive index. From the above relation we conclude that refractive index is inversely related to critical angle. Therefore as the refractive index increases, the critical angle decreases. Now light having frequency greater than green light will suffer ...
... where c is the critical angle and is the refractive index. From the above relation we conclude that refractive index is inversely related to critical angle. Therefore as the refractive index increases, the critical angle decreases. Now light having frequency greater than green light will suffer ...
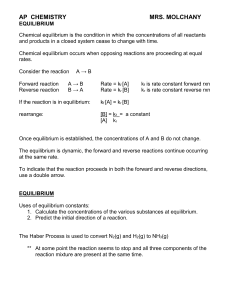
equilibrium - TeacherWeb
... 1. Tabulate the known initial and equilibrium concentrations of all species involved in the equilibrium. 2. For those species for which both the initial and equilibrium concentrations are known, calculate the change in concentration that occurs as the system reaches equilibrium. 3. Use the stoichiom ...
... 1. Tabulate the known initial and equilibrium concentrations of all species involved in the equilibrium. 2. For those species for which both the initial and equilibrium concentrations are known, calculate the change in concentration that occurs as the system reaches equilibrium. 3. Use the stoichiom ...
Stabilization of Quinapril by Incorporating Hydrogen Bonding
... Roy, et al.: Stable Co-crystal of Quinapril In the present study stability of various known solvates of quinapril hydrochloride has been compared with nitromethane solvate. Nitromethane solvate was found to be more stable compared to other known solvates. Single crystal X-ray diffraction analysis of ...
... Roy, et al.: Stable Co-crystal of Quinapril In the present study stability of various known solvates of quinapril hydrochloride has been compared with nitromethane solvate. Nitromethane solvate was found to be more stable compared to other known solvates. Single crystal X-ray diffraction analysis of ...
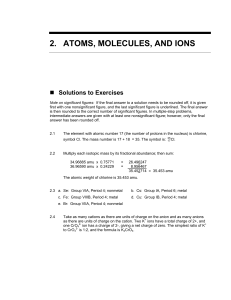
2.ATOMS, MOLECULES, AND IONS
... Some of the advantages of the Stock system are that more than two different ions of the same metal can be named with this system. In the former (older) system, a new suffix other than -ic and -ous must be established and/or memorized. ...
... Some of the advantages of the Stock system are that more than two different ions of the same metal can be named with this system. In the former (older) system, a new suffix other than -ic and -ous must be established and/or memorized. ...
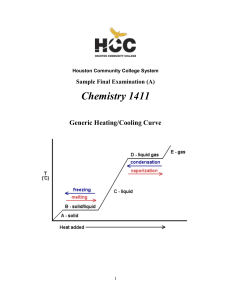
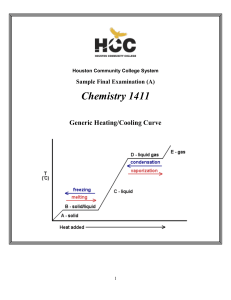
CHEM-1411 Final Practice Exam
... Since there are a total of four atoms plus lone pairs (four “electron domains”) around the central sulfur, the overall geometry is tetrahedral and the molecular geometry is trigonal pyramidal. The hybridization of the sulfur atom in the first structure is therefore sp3. However, the sulfur is not s ...
... Since there are a total of four atoms plus lone pairs (four “electron domains”) around the central sulfur, the overall geometry is tetrahedral and the molecular geometry is trigonal pyramidal. The hybridization of the sulfur atom in the first structure is therefore sp3. However, the sulfur is not s ...
CHE 1031 Lab Manual
... Experimental chemistry is inherently dangerous. Many experiments can be hazardous unless the scientist is aware of the nature of the materials being used and uses careful technique, thinking steps through bef ...
... Experimental chemistry is inherently dangerous. Many experiments can be hazardous unless the scientist is aware of the nature of the materials being used and uses careful technique, thinking steps through bef ...
Structure and Properties of Matter
... are aluminium sliver, water, carbon dioxide, nitrogen, oxygen etc. Substances differ from one another in composition and can be identified by their properties like colour, smell, taste, appearance, etc. Aluminium has uniform composition. Similarly water has uniform composition. No doubt there are al ...
... are aluminium sliver, water, carbon dioxide, nitrogen, oxygen etc. Substances differ from one another in composition and can be identified by their properties like colour, smell, taste, appearance, etc. Aluminium has uniform composition. Similarly water has uniform composition. No doubt there are al ...
AP Chemistry: Course Introduction Sheet
... Example: 101 has 3 sig. fig. and 34055 has 5 sig. fig. 2. When the measurement is a whole number ending with 0’s, the 0’s are never significant. Example: 210 has 2 sig. fig. and 71,000,000 also has 2 sig. fig. 3. When the measurement is less than a whole number, the 0’s between the decimal and other ...
... Example: 101 has 3 sig. fig. and 34055 has 5 sig. fig. 2. When the measurement is a whole number ending with 0’s, the 0’s are never significant. Example: 210 has 2 sig. fig. and 71,000,000 also has 2 sig. fig. 3. When the measurement is less than a whole number, the 0’s between the decimal and other ...
File - IB CHEM NINJA
... rate of reaction (b) with time in establishing a chemical equilibrium In an equilibrium all of the species involved, both reactants and products, are present at a constant concentration. As a consequence, macroscopic properties of the system (that is those that can be observed or measured, such as i ...
... rate of reaction (b) with time in establishing a chemical equilibrium In an equilibrium all of the species involved, both reactants and products, are present at a constant concentration. As a consequence, macroscopic properties of the system (that is those that can be observed or measured, such as i ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.