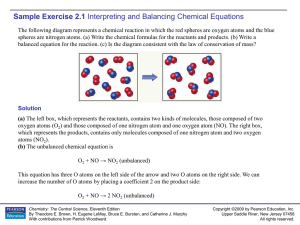
Chemistry 11th
... (ii) The oxides of alkali and alkaline earth metal dissolve in water to form their respective hydroxides. These oxides are strong bases. However, the oxides of alkali metals are more basic than those of alkaline earth metals. This is because the ionization enthalpy of alkali metals is lower. The e ...
... (ii) The oxides of alkali and alkaline earth metal dissolve in water to form their respective hydroxides. These oxides are strong bases. However, the oxides of alkali metals are more basic than those of alkaline earth metals. This is because the ionization enthalpy of alkali metals is lower. The e ...
STOICHIOMETRY REVIEW WORKSHEET
... Part 2: Solve the following stoichiometry grams-grams problems: 1) The combustion of a sample of butane, C4H10 (lighter fluid), produced 2.46 grams of water. C4H10 + ...
... Part 2: Solve the following stoichiometry grams-grams problems: 1) The combustion of a sample of butane, C4H10 (lighter fluid), produced 2.46 grams of water. C4H10 + ...
Process- och Komponentteknologi FFF110
... Silicon - Silicon is semiconducting, and has a stable high-quality oxide with good insulating properties. It is a good barrier for diffusion. It has a wider bandgap than Ge, and transistors can therefore be operated at higher temperatures. Silicon is very cheap and can be highly purified. It is not ...
... Silicon - Silicon is semiconducting, and has a stable high-quality oxide with good insulating properties. It is a good barrier for diffusion. It has a wider bandgap than Ge, and transistors can therefore be operated at higher temperatures. Silicon is very cheap and can be highly purified. It is not ...
CH4 Student Revision Guides pdf | GCE AS/A
... In an alkene such as ethene, C2H4, the double bond prevents this rotation. There is no rotation around the carbon-carbon double bond and the molecule is confined to a planar shape. This means that in compounds such as 1,2-dichloroethene, represented by the ball and stick diagrams below, two forms ar ...
... In an alkene such as ethene, C2H4, the double bond prevents this rotation. There is no rotation around the carbon-carbon double bond and the molecule is confined to a planar shape. This means that in compounds such as 1,2-dichloroethene, represented by the ball and stick diagrams below, two forms ar ...
- Career Point Kota
... (i) In case of transition element ns and (n – 1)d electron both participate in bonding due to less energy difference when ns electron take part in bonding they exhibit lower oxidation state while in case of higher O.S. (n – 1)d and ns eΘ both involve in bonding. (ii) Transition element are hard & ha ...
... (i) In case of transition element ns and (n – 1)d electron both participate in bonding due to less energy difference when ns electron take part in bonding they exhibit lower oxidation state while in case of higher O.S. (n – 1)d and ns eΘ both involve in bonding. (ii) Transition element are hard & ha ...
surface chemistry - einstein classes
... Peptization : The process of converting precipitate into colloidal solution by shaking it with dispersion medium in the presence of (small amount of) electrolyte. The electrolyte used for this purpose is called peptizing agent or stablizing agent. During peptization, the precipitate absorbs one of t ...
... Peptization : The process of converting precipitate into colloidal solution by shaking it with dispersion medium in the presence of (small amount of) electrolyte. The electrolyte used for this purpose is called peptizing agent or stablizing agent. During peptization, the precipitate absorbs one of t ...
Rotational−Vibrational Levels of Diatomic Molecules Represented
... ) ω2e /4ωexe, is applied. However, the Birge-Sponer approximation can lead to errors in values of D that can be as large as 30%). In this work, we calculate β from the relationship 6 (Tables 1 and 2) because the constants ωe and D available in literature are usually more accurate than the anharmonic ...
... ) ω2e /4ωexe, is applied. However, the Birge-Sponer approximation can lead to errors in values of D that can be as large as 30%). In this work, we calculate β from the relationship 6 (Tables 1 and 2) because the constants ωe and D available in literature are usually more accurate than the anharmonic ...
Investigating the kinetic mechanisms of the oxygen
... exists. The formation of Li2O is kinetically limited, and the major discharge product is Li2O2, even at standard potentials more negative than 2.91 V. In theoretical and experimental studies using density functional theory (DFT) and spectroscopic measurements, respectively, Li2O2 production was repo ...
... exists. The formation of Li2O is kinetically limited, and the major discharge product is Li2O2, even at standard potentials more negative than 2.91 V. In theoretical and experimental studies using density functional theory (DFT) and spectroscopic measurements, respectively, Li2O2 production was repo ...
Chemistry 133 Problem Set Introduction
... 1.55 Determine which of the following quantities are measured and which are exact. (a) A book has a mass of 1.000 kg. (b) There are 1000 g in a kilogram. (c) There are 12 inches in a foot. (d) Thirty-seven people entered a room. (e) A football stadium holds 80,000 people. 1.56 How many significant ...
... 1.55 Determine which of the following quantities are measured and which are exact. (a) A book has a mass of 1.000 kg. (b) There are 1000 g in a kilogram. (c) There are 12 inches in a foot. (d) Thirty-seven people entered a room. (e) A football stadium holds 80,000 people. 1.56 How many significant ...
GCE Getting Started - Edexcel
... Reinforcing knowledge, skills and literacy in chemistry From our research, we know that it is easy for teachers to fall into the trap of going over work that has already been covered extensively at KS4. This may be because of a feeling that during the summer break students have forgotten what they h ...
... Reinforcing knowledge, skills and literacy in chemistry From our research, we know that it is easy for teachers to fall into the trap of going over work that has already been covered extensively at KS4. This may be because of a feeling that during the summer break students have forgotten what they h ...
Math Review
... exponent will always be a whole number in chemistry, although the exponent can be fractional number in other disciplines. There are three rules for using scientific notation: Rule 1: To express a number in scientific notation, you move the decimal point to the position such that there is one nonzero ...
... exponent will always be a whole number in chemistry, although the exponent can be fractional number in other disciplines. There are three rules for using scientific notation: Rule 1: To express a number in scientific notation, you move the decimal point to the position such that there is one nonzero ...
Hydrogen: An Assessment of Its Potential for Energy Use
... the potential for hydrogen energy to affect US energy applications from economic and national security standpoints is driven primarily by the possibility of developing practical transportation solutions. Hydrogen‐powered vehicles will use a hydrogen fuel cell or hydrogen co ...
... the potential for hydrogen energy to affect US energy applications from economic and national security standpoints is driven primarily by the possibility of developing practical transportation solutions. Hydrogen‐powered vehicles will use a hydrogen fuel cell or hydrogen co ...
Periodic table, elements and physical chemistry
... (a) The student wants to prepare a standard solution of 2-hydroxypropanoic acid that has a pH of 2.19. Plan how the student could prepare 250 cm3 of this standard solution from solid 2-hydroxypropanoic acid. In your answer you should provide detail of the practical procedure that would be carried ou ...
... (a) The student wants to prepare a standard solution of 2-hydroxypropanoic acid that has a pH of 2.19. Plan how the student could prepare 250 cm3 of this standard solution from solid 2-hydroxypropanoic acid. In your answer you should provide detail of the practical procedure that would be carried ou ...
Mass Mass - White Plains Public Schools
... Number of moles of sulfuric acid (unknown) = 0.229 mole 3. Change the number of moles of the unknown to mass with the formula; # of moles of sulfuric acid = 0.229 mole mass of sulfuric acid = 22.5 g 22.5 grams of sulfuric acid to completely react with 15.0 grams of zinc. ...
... Number of moles of sulfuric acid (unknown) = 0.229 mole 3. Change the number of moles of the unknown to mass with the formula; # of moles of sulfuric acid = 0.229 mole mass of sulfuric acid = 22.5 g 22.5 grams of sulfuric acid to completely react with 15.0 grams of zinc. ...
2 - TestBankTop
... Statement (a) is the best statement regarding molecular compounds. Although you may have wanted to classify Br2 as a molecular compound, it is an element and not a compound. Regarding statement (b), quite a few molecular compounds exit that don’t contain carbon. Water and the nitrogen oxides associa ...
... Statement (a) is the best statement regarding molecular compounds. Although you may have wanted to classify Br2 as a molecular compound, it is an element and not a compound. Regarding statement (b), quite a few molecular compounds exit that don’t contain carbon. Water and the nitrogen oxides associa ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.