AP Chemistry - Oak Park Unified School District
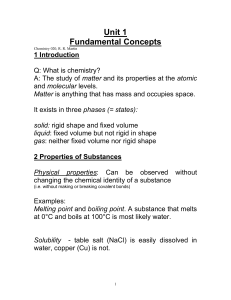
... are supported by repeatable (3) evidence. Measurements are made using the metric system, where the standard units are called (4) units, which are based on the meter, kilogram, and second as the basic units of length, mass, and time, respectively. The SI temperature scale is the (5) scale, although t ...
... are supported by repeatable (3) evidence. Measurements are made using the metric system, where the standard units are called (4) units, which are based on the meter, kilogram, and second as the basic units of length, mass, and time, respectively. The SI temperature scale is the (5) scale, although t ...
2 - TEST BANK 360
... Statement (a) is the best statement regarding molecular compounds. Although you may have wanted to classify Br2 as a molecular compound, it is an element and not a compound. Regarding statement (b), quite a few molecular compounds exit that don’t contain carbon. Water and the nitrogen oxides associa ...
... Statement (a) is the best statement regarding molecular compounds. Although you may have wanted to classify Br2 as a molecular compound, it is an element and not a compound. Regarding statement (b), quite a few molecular compounds exit that don’t contain carbon. Water and the nitrogen oxides associa ...
physical setting chemistry
... Acid rain lowers the pH in ponds and lakes and over time can cause the death of some aquatic life. Acid rain is caused in large part by the burning of fossil fuels in power plants and by gasoline-powered vehicles. The acids commonly associated with acid rain are sulfurous acid, sulfuric acid, and ni ...
... Acid rain lowers the pH in ponds and lakes and over time can cause the death of some aquatic life. Acid rain is caused in large part by the burning of fossil fuels in power plants and by gasoline-powered vehicles. The acids commonly associated with acid rain are sulfurous acid, sulfuric acid, and ni ...
Ch. 11-12 Supplements
... b. How many grams of each product will be formed? c. How many grams of excess reactant remain? 4) a. Write the balanced equation for lithium metal, Li, reacting with aqueous copper (II) nitrate, Cu(NO3)2, to produce copper metal, Cu, and aqueous lithium nitrate, LiNO3. b. If 15.0 grams of lithium re ...
... b. How many grams of each product will be formed? c. How many grams of excess reactant remain? 4) a. Write the balanced equation for lithium metal, Li, reacting with aqueous copper (II) nitrate, Cu(NO3)2, to produce copper metal, Cu, and aqueous lithium nitrate, LiNO3. b. If 15.0 grams of lithium re ...
Chemical Equilibrium - Department of Chemistry
... If we increase the total pressure at constant volume by adding an inert gas or another gas not involved in the equilibrium, the partial pressure of each species in the equilibrium will not change…therefore the reaction quotient will not change. The system will not respond to changes in total pressur ...
... If we increase the total pressure at constant volume by adding an inert gas or another gas not involved in the equilibrium, the partial pressure of each species in the equilibrium will not change…therefore the reaction quotient will not change. The system will not respond to changes in total pressur ...
Chapter 7 – Chemical Formulas and Chemical
... polyatomic ions. These are groups of atoms bonded together covalently. What makes them different is that they carry a charge as a group. Ex: OH-, SO4-2, NH4+ These polyatomic atoms like many other formulas have common names that do not state the chemical composition. The common name for dihydrogen m ...
... polyatomic ions. These are groups of atoms bonded together covalently. What makes them different is that they carry a charge as a group. Ex: OH-, SO4-2, NH4+ These polyatomic atoms like many other formulas have common names that do not state the chemical composition. The common name for dihydrogen m ...
x - SharpSchool
... In a 500 mL stainless steel reaction vessel at 900C, carbon monoxide and water vapour react to produce carbon dioxide and hydrogen. Evidence indicates that this reaction establishes an equilibrium with only partial conversion of reactants to products. Initially, 2.00 mol of each reactant is placed ...
... In a 500 mL stainless steel reaction vessel at 900C, carbon monoxide and water vapour react to produce carbon dioxide and hydrogen. Evidence indicates that this reaction establishes an equilibrium with only partial conversion of reactants to products. Initially, 2.00 mol of each reactant is placed ...
PDF - ACS Publications - American Chemical Society
... In eq 29, t is the time elapsed from the beginning of the TP experiment and extends to the relaxation period to the point where the cell retrieves its equilibrium (Δcb = 0). During the TP experiment, this is the potential across the cell and not the concentration difference which is recorded. For suffi ...
... In eq 29, t is the time elapsed from the beginning of the TP experiment and extends to the relaxation period to the point where the cell retrieves its equilibrium (Δcb = 0). During the TP experiment, this is the potential across the cell and not the concentration difference which is recorded. For suffi ...
File
... Accept room temperature as an answer if platinum or palladium used. the enthalpy change when (one mole of) the gaseous bond is broken (or formed) / X–Y(g) → X(g) + Y(g) / X(g) + Y(g) → X–Y(g); averaged for the same bond in a number of similar compounds / OWTTE; ...
... Accept room temperature as an answer if platinum or palladium used. the enthalpy change when (one mole of) the gaseous bond is broken (or formed) / X–Y(g) → X(g) + Y(g) / X(g) + Y(g) → X–Y(g); averaged for the same bond in a number of similar compounds / OWTTE; ...
Homework Booklet [4,S]
... 3) Draw a labelled diagram of a fractionating column giving two uses for each of the fractions obtained. [4] 4) The petroleum fraction contains a hydrocarbon with the molecular formula, C7H16. i) What is a hydrocarbon? ii) Write a balanced equation for the complete combustion of C7H16. iii) What wou ...
... 3) Draw a labelled diagram of a fractionating column giving two uses for each of the fractions obtained. [4] 4) The petroleum fraction contains a hydrocarbon with the molecular formula, C7H16. i) What is a hydrocarbon? ii) Write a balanced equation for the complete combustion of C7H16. iii) What wou ...
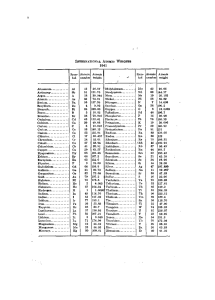
INTEKNATIONAL ATOMIC WEIGHTS Aluminum... Antimony..., Argon
... This revision introduces many new experiments and revises others in an attempt to keep abreast of the rapid developments in physical chemistry. Some of the former experiments have been eliminated or expanded because they have found their way into earlier courses and are already known to students, wh ...
... This revision introduces many new experiments and revises others in an attempt to keep abreast of the rapid developments in physical chemistry. Some of the former experiments have been eliminated or expanded because they have found their way into earlier courses and are already known to students, wh ...
carboxylic acid
... limited to use with primary and some secondary alkyl halides. The second method involves formation of a Grignard reagent and is therefore limited to use with organic halides that have no acidic hydrogens or reactive functional groups. In the present instance, either method would work well. ...
... limited to use with primary and some secondary alkyl halides. The second method involves formation of a Grignard reagent and is therefore limited to use with organic halides that have no acidic hydrogens or reactive functional groups. In the present instance, either method would work well. ...
57 estonian national chemistry olympiad
... of B can be written as AX3. If gas B is heated in hydrogen atmosphere, elementary compound A If formed. A is also formed by thermal decomposition of iodide AI3 and in reaction of oxide A2O3 with magnesium. The second product in three given reactions of A formation are strong acid C, elementary subst ...
... of B can be written as AX3. If gas B is heated in hydrogen atmosphere, elementary compound A If formed. A is also formed by thermal decomposition of iodide AI3 and in reaction of oxide A2O3 with magnesium. The second product in three given reactions of A formation are strong acid C, elementary subst ...
- Kendriya Vidyalaya No. 2 Raipur
... iii) Reaction with Non -metallic oxides – These oxides are generally acidic in nature. They react with bases to form salt and water. 2NaOH + CO2 Na2CO3 + H2O 7) PH Scale: The concentration of hydrogen ion in solution is expressed in terms of pH. The pH of a solution is defined as the negative loga ...
... iii) Reaction with Non -metallic oxides – These oxides are generally acidic in nature. They react with bases to form salt and water. 2NaOH + CO2 Na2CO3 + H2O 7) PH Scale: The concentration of hydrogen ion in solution is expressed in terms of pH. The pH of a solution is defined as the negative loga ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.
















![Homework Booklet [4,S]](http://s1.studyres.com/store/data/010355871_1-63c750e3d1b58eaaebbb3f5d45651c44-300x300.png)