Calculations with Chemical Formulas and Equations
... a. The limiting reactant when cooking with a gas grill would be the propane. This makes sense since propane is the material that you must purchase in order to cook your food. b. Since the chemical reaction only requires propane and oxygen, if the grill will not light with ample propane present, then ...
... a. The limiting reactant when cooking with a gas grill would be the propane. This makes sense since propane is the material that you must purchase in order to cook your food. b. Since the chemical reaction only requires propane and oxygen, if the grill will not light with ample propane present, then ...
Balancing a Chemical Equation
... Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in order to balance the equation. Check your answer to see if: The numbers of atoms on both sides of the equation are now balanced. The coefficients ar ...
... Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in order to balance the equation. Check your answer to see if: The numbers of atoms on both sides of the equation are now balanced. The coefficients ar ...
balancing eqns teacher
... compound. Subscripts are determined by the valence electrons (charges for ionic or sharing for covalent) Think ...
... compound. Subscripts are determined by the valence electrons (charges for ionic or sharing for covalent) Think ...
PPT CH 8
... for Acids and Bases • pH scale - a scale that indicates the acidity or basicity of a solution – Ranges from 0 (very acidic) to 14 (very basic) ...
... for Acids and Bases • pH scale - a scale that indicates the acidity or basicity of a solution – Ranges from 0 (very acidic) to 14 (very basic) ...
Conservation of Energy in chemical reactions, Hess`s Law
... What kind of change is occurring this time, and what elements are involved? What is the critical difference? What would H be in the second reaction? _____ Why does this make sense? ...
... What kind of change is occurring this time, and what elements are involved? What is the critical difference? What would H be in the second reaction? _____ Why does this make sense? ...
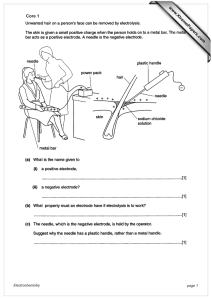
electrochemistry - einstein classes
... A devide that converts chemical energy into electrical energy. The cell is based on the principle of indirect redox reactions, i.e, the oxidation and reduction reactions takes place in different container. The electrochemical cells (galvanic or voltaic) consists of two half cells connected with each ...
... A devide that converts chemical energy into electrical energy. The cell is based on the principle of indirect redox reactions, i.e, the oxidation and reduction reactions takes place in different container. The electrochemical cells (galvanic or voltaic) consists of two half cells connected with each ...
Chapter Ten
... 10.16 Acidity and Basicity of Salt Solutions ► Salt solutions can be neutral, acidic, or basic, depending on the ions present, because some ions react with water to produce H+ and some ions react with water to produce OH-. ► To predict the acidity of a salt solution, it is convenient to classify sa ...
... 10.16 Acidity and Basicity of Salt Solutions ► Salt solutions can be neutral, acidic, or basic, depending on the ions present, because some ions react with water to produce H+ and some ions react with water to produce OH-. ► To predict the acidity of a salt solution, it is convenient to classify sa ...
Chem EOC Review Cumulative Free Response
... 72) Atoms form a mostly covalent bond if they have a _________ difference in electronegativity. 73) A _______________ compound is one that contains covalent bonds. 74) List the 7 diatomic molecules. 75) What type of bond exists in an N2 molecule? ______________ ______________ 76) Name a diatomic tha ...
... 72) Atoms form a mostly covalent bond if they have a _________ difference in electronegativity. 73) A _______________ compound is one that contains covalent bonds. 74) List the 7 diatomic molecules. 75) What type of bond exists in an N2 molecule? ______________ ______________ 76) Name a diatomic tha ...
2011-2012 Summer Packet - Tenafly Public Schools
... compounds or into elements, but this requires chemical methods such as reaction with acid, electrolysis, or the input of heat energy. A few compounds, like nitroglycerine, decompose into simpler substances quite readily without much energy input. When a compound is made from elements, the compound t ...
... compounds or into elements, but this requires chemical methods such as reaction with acid, electrolysis, or the input of heat energy. A few compounds, like nitroglycerine, decompose into simpler substances quite readily without much energy input. When a compound is made from elements, the compound t ...
Lecture 2: Introduction to Proteins
... B. For that same carboxyl group, what fraction, or what percentage, of the total (population of all the molecules in solution) is present in the form of the ACID (COOH) at pH 5.0? What fraction of the total is present in the form of the BASE (COO–) at pH 5? 3. Suppose that about 1% of the molecules ...
... B. For that same carboxyl group, what fraction, or what percentage, of the total (population of all the molecules in solution) is present in the form of the ACID (COOH) at pH 5.0? What fraction of the total is present in the form of the BASE (COO–) at pH 5? 3. Suppose that about 1% of the molecules ...
Chemistry 199 - Oregon State chemistry
... 5730 years and the carbon-14 in our bodies is decaying. So as long as we eat and breathe we are replenishing our carbon-14 to keep the level constant. A plant or mammal does not replenish its carbon-14 after death. Euphemism: George Washington is not in the carbon cycle. Fossils have less C-14 than ...
... 5730 years and the carbon-14 in our bodies is decaying. So as long as we eat and breathe we are replenishing our carbon-14 to keep the level constant. A plant or mammal does not replenish its carbon-14 after death. Euphemism: George Washington is not in the carbon cycle. Fossils have less C-14 than ...
Module Seven - DePauw University
... The examples and problems in Modules 5 and 6 assume that a compound’s amount is provided in grams. This is not surprising as mass is perhaps the most fundamental of measurements (you might recall that it is one of seven base quantities in the SI system) and it is the most accurate and precise method ...
... The examples and problems in Modules 5 and 6 assume that a compound’s amount is provided in grams. This is not surprising as mass is perhaps the most fundamental of measurements (you might recall that it is one of seven base quantities in the SI system) and it is the most accurate and precise method ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.