Ionic Bonding
... noble gas core, they are not found in ionic compounds with a noble gas core (thus they may have color). Some examples which can form a noble gas core (and be colorless): Ag: [Kr]5s14d10 Ag+ [Kr]4d10 Compound: AgCl Cd: [Kr]5s24d10 Cd2+ [Kr]4d10 Compound: CdS The valence electrons do not adhere to the ...
... noble gas core, they are not found in ionic compounds with a noble gas core (thus they may have color). Some examples which can form a noble gas core (and be colorless): Ag: [Kr]5s14d10 Ag+ [Kr]4d10 Compound: AgCl Cd: [Kr]5s24d10 Cd2+ [Kr]4d10 Compound: CdS The valence electrons do not adhere to the ...
2013 Avogadro Exam
... This exam is being written by several thousand students. Please be sure that you follow the instructions below. We'll send your teacher a report on your performance. Top performers are eligible for a prize. The names of the top 200 students will be published in the September issue of Chem 13 News. ...
... This exam is being written by several thousand students. Please be sure that you follow the instructions below. We'll send your teacher a report on your performance. Top performers are eligible for a prize. The names of the top 200 students will be published in the September issue of Chem 13 News. ...
Physical chemistry 1
... • Nernst equation, standard potential, electrochemical series, potentiometric measurement, selective electrodes. • Potentiometric titration, conductivity of ions in solution, the mobility of ions. • Electron transfer in heterogeneous systems, processes at the interface of the electrode and electroly ...
... • Nernst equation, standard potential, electrochemical series, potentiometric measurement, selective electrodes. • Potentiometric titration, conductivity of ions in solution, the mobility of ions. • Electron transfer in heterogeneous systems, processes at the interface of the electrode and electroly ...
Chapter 4: Reactions in Aqueous Solution
... B) Solvent – dissolving medium. This component is always in greatest amount. C) Most chemical reactions are carried out in the liquid state or in solution. This is due to the requirement that reactant molecules or ions be highly mobile so they can interact with each other. 2) Although solutions can ...
... B) Solvent – dissolving medium. This component is always in greatest amount. C) Most chemical reactions are carried out in the liquid state or in solution. This is due to the requirement that reactant molecules or ions be highly mobile so they can interact with each other. 2) Although solutions can ...
Chapter ( 1 ) 1- Write the scientific term : 1. Simple symbolic formula
... 4. A non –homogeneous mixture produced when dissolving chalk powder in water . 5. A kind of solution which is intermediate between homogeneous and heterogeneous 6. A force appears in solid states forming the crystal lattice in which the ions are attracted 7. A force appears in the non – polar compou ...
... 4. A non –homogeneous mixture produced when dissolving chalk powder in water . 5. A kind of solution which is intermediate between homogeneous and heterogeneous 6. A force appears in solid states forming the crystal lattice in which the ions are attracted 7. A force appears in the non – polar compou ...
Carefully detach the last page. It is the Data Sheet.
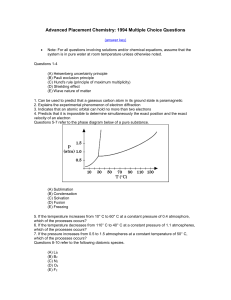
... 2 Al(s) + 6 HCl(aq) → 2 AlCl3(aq) + 3 H2(g) How many moles of H2 are produced if 17.5 moles of Al are added to a solution containing 24.8 moles of HCl? A ...
... 2 Al(s) + 6 HCl(aq) → 2 AlCl3(aq) + 3 H2(g) How many moles of H2 are produced if 17.5 moles of Al are added to a solution containing 24.8 moles of HCl? A ...
AP Exam One Retake Qualifying Assignment
... separates large substances from smaller substances the oil in a water and oil mixture Mercury Glucose C6H12O6 Gatorade cannot be broken down any further by chemical means the atmosphere Use only the best term to answer each question below. Each term may be used once, more than once, or not at all. a ...
... separates large substances from smaller substances the oil in a water and oil mixture Mercury Glucose C6H12O6 Gatorade cannot be broken down any further by chemical means the atmosphere Use only the best term to answer each question below. Each term may be used once, more than once, or not at all. a ...
Ch1-2
... Solution ----------------------------------------------------------------------------------------- = gh = 980 MwS = RT ...
... Solution ----------------------------------------------------------------------------------------- = gh = 980 MwS = RT ...
Chemical reactions
... reaction. They are written in the left term of the equation. Reaction products = substances formed in a chemical reaction. They are written in the right term of the equation Because in a chemical reaction, the nature of atoms of the substances is not changed, the chemical equations are equalized so ...
... reaction. They are written in the left term of the equation. Reaction products = substances formed in a chemical reaction. They are written in the right term of the equation Because in a chemical reaction, the nature of atoms of the substances is not changed, the chemical equations are equalized so ...
Mechanisms 3
... become a C-Cl bond. Products containing more than one halogen atom can be formed by further substitution on molecules that already contain a halogen atom. ...
... become a C-Cl bond. Products containing more than one halogen atom can be formed by further substitution on molecules that already contain a halogen atom. ...
Chap 9 Redox Review Q`s
... Which processes occur during the electrolysis of molten sodium chloride? I. Sodium and chloride ions move through the electrolyte. II. Electrons move through the external circuit. III. Oxidation takes place at the positive electrode (anode). ...
... Which processes occur during the electrolysis of molten sodium chloride? I. Sodium and chloride ions move through the electrolyte. II. Electrons move through the external circuit. III. Oxidation takes place at the positive electrode (anode). ...
Worksheet answers
... produced 1.60g CO2 and 0.819g H20. Calculate the empirical formula. Ans = C2H5 1.60 / 44.01 = 0.0364 moles CO2 = 0.0364 moles C; 0.819/18.02 = 0.0454 moles H20 = 2 x 0.0454 = 0.0908 moles H so C0.0364 H 0.0908 = CH2.5 = C2H5 (make whole number) b) Upon combustion, a 0.8009g sample of compound contai ...
... produced 1.60g CO2 and 0.819g H20. Calculate the empirical formula. Ans = C2H5 1.60 / 44.01 = 0.0364 moles CO2 = 0.0364 moles C; 0.819/18.02 = 0.0454 moles H20 = 2 x 0.0454 = 0.0908 moles H so C0.0364 H 0.0908 = CH2.5 = C2H5 (make whole number) b) Upon combustion, a 0.8009g sample of compound contai ...
Chemical Equations and Reactions
... CO2(g) + H2O(g) (not balanced) CH4(g) + 2O2(g) CO2(g) + 2H2O(g) (balanced) • Balancing: make sure that the number of moles of an element are equal on both sides of the equation • Catalyst: substance that a changes the rate of a chemical reaction but can be recovered unchanged. ...
... CO2(g) + H2O(g) (not balanced) CH4(g) + 2O2(g) CO2(g) + 2H2O(g) (balanced) • Balancing: make sure that the number of moles of an element are equal on both sides of the equation • Catalyst: substance that a changes the rate of a chemical reaction but can be recovered unchanged. ...
Review IV
... Weak and strong electrolytes (refer to lab): NEED IONS!! ; Strong acids: HCl, HBr, HI, HNO3, H2SO4, HClO4 strong base: Group IA hydroxide, group IIA hydroxide Salts soluble in H2) is strong electrolyte Soluble salts?? Salts containing Group IA cations, NH4+, , NO3Cl-, Br-, I- salts all soluble excep ...
... Weak and strong electrolytes (refer to lab): NEED IONS!! ; Strong acids: HCl, HBr, HI, HNO3, H2SO4, HClO4 strong base: Group IA hydroxide, group IIA hydroxide Salts soluble in H2) is strong electrolyte Soluble salts?? Salts containing Group IA cations, NH4+, , NO3Cl-, Br-, I- salts all soluble excep ...
Key - GCC
... 14. List the three types of redox reactions and describe how you can identify them: Combination (two or more elements or compounds combine to form a single product); Decomposition (one compound decomposes, usually with heat, to give off a gas and another substance); Singlereplacement (one element an ...
... 14. List the three types of redox reactions and describe how you can identify them: Combination (two or more elements or compounds combine to form a single product); Decomposition (one compound decomposes, usually with heat, to give off a gas and another substance); Singlereplacement (one element an ...
Homework Solutions Week 6
... According to Box 9-1, the source of calcium in the rivers is the mineral calcite, which dissolves by reacting with carbon dioxide in the river waver according to the equation: CaCO3(s) + CO2(aq) + H2O < == > Ca2+ + 2 HCO3If the predominate product is bicarbonate and not carbonate or carbonic acid,th ...
... According to Box 9-1, the source of calcium in the rivers is the mineral calcite, which dissolves by reacting with carbon dioxide in the river waver according to the equation: CaCO3(s) + CO2(aq) + H2O < == > Ca2+ + 2 HCO3If the predominate product is bicarbonate and not carbonate or carbonic acid,th ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.