Measurements/Unit Cancellation/Significant Figures 1. When
... Formula mass: The sum of the atomic masses (atomic weights in amu) of the atomic species as given in the formula of the compound Hydrate: A compound in which a specific number of water molecules are associated with each formula unit. Hydrated ion: An ion in which a specific number of water molecules ...
... Formula mass: The sum of the atomic masses (atomic weights in amu) of the atomic species as given in the formula of the compound Hydrate: A compound in which a specific number of water molecules are associated with each formula unit. Hydrated ion: An ion in which a specific number of water molecules ...
50 Frequently Forgotten Facts
... 21) At STP, the liquids on the Periodic Table are Br and Hg. The gases are N, Cl, H, O, F and the Noble Gases. All other elements are solids. [Periodic Table] a) Which element on the Periodic Table is a nonmetallic liquid at STP?____________________________ b) Which element at STP is a liquid that ...
... 21) At STP, the liquids on the Periodic Table are Br and Hg. The gases are N, Cl, H, O, F and the Noble Gases. All other elements are solids. [Periodic Table] a) Which element on the Periodic Table is a nonmetallic liquid at STP?____________________________ b) Which element at STP is a liquid that ...
Charging of Oil-Water Interfaces Due to Spontaneous Adsorption of
... In spite of the experimental evidence of negative charging of the oil-water interface, and the importance of this phenomenon for colloid behavior and stability, the origin of the charge is by no means well understood.19 The only argument used to support the adsorption of hydroxyl ions as a source fo ...
... In spite of the experimental evidence of negative charging of the oil-water interface, and the importance of this phenomenon for colloid behavior and stability, the origin of the charge is by no means well understood.19 The only argument used to support the adsorption of hydroxyl ions as a source fo ...
Hints for Names and Formulas (Ch. 4 in Zumdahl Chemistry)
... (7) all polyatomic ions have atoms that are bonded covalently, but total charge does not equal zero (8) most organic molecules are named according to systematic IUPAC nomenclature rules ● IUPAC stands for the International Union of Pure and Applied Chemistry committee (9) most covalent inorganic mol ...
... (7) all polyatomic ions have atoms that are bonded covalently, but total charge does not equal zero (8) most organic molecules are named according to systematic IUPAC nomenclature rules ● IUPAC stands for the International Union of Pure and Applied Chemistry committee (9) most covalent inorganic mol ...
Chemistry 116: General Chemistry
... The reaction is faster at higher temperatures. The reaction has only one type of reactant. The rate remains constant when the reactant concentration is doubled. The reaction slows down as time goes on. The half life remains constant as time goes on. ...
... The reaction is faster at higher temperatures. The reaction has only one type of reactant. The rate remains constant when the reactant concentration is doubled. The reaction slows down as time goes on. The half life remains constant as time goes on. ...
Chapter 1
... a) rice pudding Heterogeneous mixture b) seawater Homogeneous mixture unless there are undissolved particles such as sand, then heterogeneous c) magnesium Element d) gasoline Homogeneous mixture ...
... a) rice pudding Heterogeneous mixture b) seawater Homogeneous mixture unless there are undissolved particles such as sand, then heterogeneous c) magnesium Element d) gasoline Homogeneous mixture ...
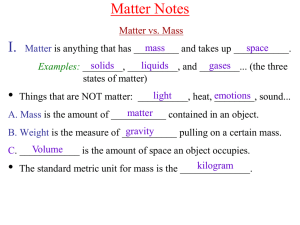
Chem A Week 2 Matter Notes
... Vapor is a term used for the gaseous form of a substance liquid or _______ solid at room temperature.) that is normally a ________ Example: _______ water vapor (steam) ...
... Vapor is a term used for the gaseous form of a substance liquid or _______ solid at room temperature.) that is normally a ________ Example: _______ water vapor (steam) ...
Lecture 11 - U of L Class Index
... CaO, were alkaline according to the experimental tests of the alchemists: they had a bitter taste and could be shown to neutralize acids. Group 1 compounds, however, melted in a fire or combined with the clay containers in which they were heated. Melting points of most Group 2 compounds are very hig ...
... CaO, were alkaline according to the experimental tests of the alchemists: they had a bitter taste and could be shown to neutralize acids. Group 1 compounds, however, melted in a fire or combined with the clay containers in which they were heated. Melting points of most Group 2 compounds are very hig ...
- International Journal of Multidisciplinary Research and
... 4 moles of hydrogen in total and one mole of carbon dioxide which is purified later in a pressure swing adsorption unit. After pure hydrogen is extracted from the PSA, it's supplied to a fuel cell that typically produce hydrogen. An experiment has been conducted to measure the electric current gener ...
... 4 moles of hydrogen in total and one mole of carbon dioxide which is purified later in a pressure swing adsorption unit. After pure hydrogen is extracted from the PSA, it's supplied to a fuel cell that typically produce hydrogen. An experiment has been conducted to measure the electric current gener ...
Multiple Choice Math Practice File
... o they are generally lined up by decimal points, even though this does not make the number list “straight” Allows you to see decimal points and significant figures easier. Look for the word approximate in the question, if there, you can use estimation to help arrive at the answer. Strategies on ...
... o they are generally lined up by decimal points, even though this does not make the number list “straight” Allows you to see decimal points and significant figures easier. Look for the word approximate in the question, if there, you can use estimation to help arrive at the answer. Strategies on ...
Name______________________ Period________
... 30. According to the Dual Nature of Light, light acts as a __________________ and a _________________. ...
... 30. According to the Dual Nature of Light, light acts as a __________________ and a _________________. ...
IChO 35 Theoretical Exam
... (a) HF boils at a higher temperature than HCl. Y N (b) HBr boils at a lower temperature than HI Y N (c) Pure HI can be produced by reacting concentrated sulfuric acid with KI. Y N (d) Ammonia solutions are buffer solutions because they contain the conjugate pair NH3 – NH4+. Y N (e) Pure water at 80° ...
... (a) HF boils at a higher temperature than HCl. Y N (b) HBr boils at a lower temperature than HI Y N (c) Pure HI can be produced by reacting concentrated sulfuric acid with KI. Y N (d) Ammonia solutions are buffer solutions because they contain the conjugate pair NH3 – NH4+. Y N (e) Pure water at 80° ...
AP Review – Life and Chemistry Name: Date: ___B_ 1. The atomic
... Calcium’s electrons in orbitals are shown to the left. Notice how the two electrons in the valence shell (outermost shell) are paired? This is done sometimes when only two electrons are in the valence shell – it helps to make sure you don’t “lose them” in the diagram by separating them. To draw ...
... Calcium’s electrons in orbitals are shown to the left. Notice how the two electrons in the valence shell (outermost shell) are paired? This is done sometimes when only two electrons are in the valence shell – it helps to make sure you don’t “lose them” in the diagram by separating them. To draw ...
E4 ION DIFFUSION
... Amount of substance Mass is a common way of specifying how much material you have, but for some purposes it is much more pertinent to think in term of how many particles you have. Since the atoms or molecules of different substances have different masses, equal masses of different substances contain ...
... Amount of substance Mass is a common way of specifying how much material you have, but for some purposes it is much more pertinent to think in term of how many particles you have. Since the atoms or molecules of different substances have different masses, equal masses of different substances contain ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.