Notes. - Net Start Class
... Chemical properties describe the reaction of a substance with other material or the reaction within the substance itself. Lack of chemical reactivity is also important. ...
... Chemical properties describe the reaction of a substance with other material or the reaction within the substance itself. Lack of chemical reactivity is also important. ...
v` mf - EngineeringDuniya.com
... the force of gravity in CCl4 (S.G 1.594) @ 20ºC. The dia of sphalerite particles is 0.004”. The volume fraction of sphalerite in CCl4 is 0.2. What is the settling velocity of sphalerite? Assume Stoke’s law is valid. Richardson zaki index is = 4.1. Take viscosity is 1.03 cP • ut=0.0131m/s • us=5.2mm/ ...
... the force of gravity in CCl4 (S.G 1.594) @ 20ºC. The dia of sphalerite particles is 0.004”. The volume fraction of sphalerite in CCl4 is 0.2. What is the settling velocity of sphalerite? Assume Stoke’s law is valid. Richardson zaki index is = 4.1. Take viscosity is 1.03 cP • ut=0.0131m/s • us=5.2mm/ ...
States of Matter WebQuest
... 27. Use the chart to identify the state of matter described by the following. Many of these have more than one answer! Write solid, liquid, or gas in the spaces below. not easily compressible rigid – particles are locked into place flows easily ...
... 27. Use the chart to identify the state of matter described by the following. Many of these have more than one answer! Write solid, liquid, or gas in the spaces below. not easily compressible rigid – particles are locked into place flows easily ...
Mixture Solution Notes
... Warm Up: Dissolving 1. What does “dissolve” mean? 2. What kinds of things dissolve? 3. What do things dissolve in? ...
... Warm Up: Dissolving 1. What does “dissolve” mean? 2. What kinds of things dissolve? 3. What do things dissolve in? ...
Pollution Control - No Brain Too Small
... By use of static electricity, particles are attracted in the same way that static electricity in clothing picks up small bits of dust and lint. Electrostatic precipitators, 98 to 99 percent effective, are used instead of baghouses when the particles are suspended in very hot gases, such as in emissi ...
... By use of static electricity, particles are attracted in the same way that static electricity in clothing picks up small bits of dust and lint. Electrostatic precipitators, 98 to 99 percent effective, are used instead of baghouses when the particles are suspended in very hot gases, such as in emissi ...
World of matter - Kindle Education
... Matter is made up on small particles which are in constant motion. When you heat matter, the particles of matter absorb the heat energy and begin moving faster (in other words they gain kinetic energy). As more energy is provided the chemical bond between the particles become weaker and hence there ...
... Matter is made up on small particles which are in constant motion. When you heat matter, the particles of matter absorb the heat energy and begin moving faster (in other words they gain kinetic energy). As more energy is provided the chemical bond between the particles become weaker and hence there ...
Chapter 3 PowerPoint Notes
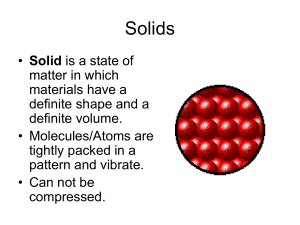
... (Takes the shape and volume of its container). • Molecules/Atoms are spread apart and can be compressed. ...
... (Takes the shape and volume of its container). • Molecules/Atoms are spread apart and can be compressed. ...
12.26MB - Stanford University
... to facilitate their display during course instruction. Permissions for publication of photographs must be requested from individual copyright holders. The source of each photograph is given below the figure and in the back of the textbook. ...
... to facilitate their display during course instruction. Permissions for publication of photographs must be requested from individual copyright holders. The source of each photograph is given below the figure and in the back of the textbook. ...
Particles - Townley Grammar School
... Describe and explain some demonstrations / examples used to demonstrate that liquids and gases exert a pressure. ...
... Describe and explain some demonstrations / examples used to demonstrate that liquids and gases exert a pressure. ...
6 Departure from thermal equilibrium
... universe, when T /Mp & α2 , this scattering process will be out of thermal equilibrium. 2. It can happen that only interaction light particles can experience involves the mediation of a heavy virtual state with mass M . Neutrinos serve as a particularly important example, as they only interact throu ...
... universe, when T /Mp & α2 , this scattering process will be out of thermal equilibrium. 2. It can happen that only interaction light particles can experience involves the mediation of a heavy virtual state with mass M . Neutrinos serve as a particularly important example, as they only interact throu ...
03 nanoparticles part 7 File - e-learning
... Advantages are: production of oxides at very small residence times, easy scale-up of the equipment and high purity; Disadvantages are: tight agglomerates formation, heterogeneous residence times and temperature profiles in the reactor, not suitable for all targets. ...
... Advantages are: production of oxides at very small residence times, easy scale-up of the equipment and high purity; Disadvantages are: tight agglomerates formation, heterogeneous residence times and temperature profiles in the reactor, not suitable for all targets. ...
POGIL: Kinetic Molecular Theory
... These particles are so small compared with the distance between them that the volume (size) of the individual particles can be assumed to be negligible (zero). The particles are in constant random motion, colliding with the walls of the container. These collisions with the walls cause the pressure e ...
... These particles are so small compared with the distance between them that the volume (size) of the individual particles can be assumed to be negligible (zero). The particles are in constant random motion, colliding with the walls of the container. These collisions with the walls cause the pressure e ...
Practice Test 2 Do equal volumes of different materials have the
... b. When heat is put into a crystal, each atom in the crystal requires more room for its vibrational motions. c. Liquid water actually shrinks a bit when its temperature increases from 0 to 4 degrees Celsius. d. The change in length of a solid can be calculated using a known coefficient of linear exp ...
... b. When heat is put into a crystal, each atom in the crystal requires more room for its vibrational motions. c. Liquid water actually shrinks a bit when its temperature increases from 0 to 4 degrees Celsius. d. The change in length of a solid can be calculated using a known coefficient of linear exp ...
Characterization of Electrospray Aerosol Generator
... climate changes [3]. Recent studies and development in aerosol instrumentation have focused on nanoparticles [1]. There is a need to know the chemical and physical compositions of and to characterize these aerosols in order to better determine what key roles nano-aerosols play in weather pattern and ...
... climate changes [3]. Recent studies and development in aerosol instrumentation have focused on nanoparticles [1]. There is a need to know the chemical and physical compositions of and to characterize these aerosols in order to better determine what key roles nano-aerosols play in weather pattern and ...
People Search for Review
... 4. What is an isotope? What makes an isotope of one element different from a different isotope of the same element? ...
... 4. What is an isotope? What makes an isotope of one element different from a different isotope of the same element? ...
Spark generated particles for nanotoxicology studies M.E. Messing1
... that the properties of a specific nano-sized material are different from the properties of the same material in bulk form, and therefore toxicology regulations often based on mass might not be relevant for nano-sized materials. In order to improve the understanding of nanotoxicology and to learn how ...
... that the properties of a specific nano-sized material are different from the properties of the same material in bulk form, and therefore toxicology regulations often based on mass might not be relevant for nano-sized materials. In order to improve the understanding of nanotoxicology and to learn how ...
science921key - Rocky View Schools
... 3. In a pure substance, all particles in the substance are identical. An example is the metal lead. A solution contains at least two different types of particles: the solvent particles (e.g., water) are more numerous, and the solute particles (e.g., sugar) are less numerous. The particles are very e ...
... 3. In a pure substance, all particles in the substance are identical. An example is the metal lead. A solution contains at least two different types of particles: the solvent particles (e.g., water) are more numerous, and the solute particles (e.g., sugar) are less numerous. The particles are very e ...
View PDF
... and microstructure of aerosol particles is now wellestablished for inferring key properties of the aerosol such as hygroscopicity, the activity of cloud condensation, the reactivity, the optical properties, etc. Aerosol particles consist of complex mixture of inorganic salts with hydrophilic and/or ...
... and microstructure of aerosol particles is now wellestablished for inferring key properties of the aerosol such as hygroscopicity, the activity of cloud condensation, the reactivity, the optical properties, etc. Aerosol particles consist of complex mixture of inorganic salts with hydrophilic and/or ...
Spectroscopy, a toolbox for structural information on aerosol particles
... CH-8093 Zurich, Switzerland [email protected] It is well known that light interacts differently with small particles compared with bulk materials or gas phase molecules, producing spectral signatures that strongly depend on particle properties, such as size, shape, or architecture. Even nanosized a ...
... CH-8093 Zurich, Switzerland [email protected] It is well known that light interacts differently with small particles compared with bulk materials or gas phase molecules, producing spectral signatures that strongly depend on particle properties, such as size, shape, or architecture. Even nanosized a ...
Aerosol

An aerosol is a colloid of fine solid particles or liquid droplets, in air or another gas. Aerosols can be natural or artificial. Examples of natural aerosols are fog, forest exudates and geyser steam. Examples of artificial aerosols are haze, dust, particulate air pollutants and smoke. The liquid or solid particles have diameter mostly smaller than 1 μm or so; larger particles with a significant settling speed make the mixture a suspension, but the distinction is not clear-cut. In general conversation, aerosol usually refers to an aerosol spray that delivers a consumer product from a can or similar container. Other technological applications of aerosols include dispersal of pesticides, medical treatment of respiratory illnesses, and combustion technology. Diseases can also spread by means of small droplets in the breath, also called aerosols.Aerosol science covers generation and removal of aerosols, technological application of aerosols, effects of aerosols on the environment and people, and a wide variety of other topics.