Stage 2 Chemistry Intended Student Learning 2014
... Draw a line of best fit through a series of points on a graph such that the plotted points are scattered evenly above and below the line of best fit. ...
... Draw a line of best fit through a series of points on a graph such that the plotted points are scattered evenly above and below the line of best fit. ...
Discover Chemical Changes - gk-12
... Burning – the chemical process of combustion, which is an exothermic chemical reaction between a substance, in this case the paper, which acts as the fuel, and a gas, in this case the air, which is the oxidizer (i.e. the gas must contain oxygen). As we all know from experience, combustion, or burnin ...
... Burning – the chemical process of combustion, which is an exothermic chemical reaction between a substance, in this case the paper, which acts as the fuel, and a gas, in this case the air, which is the oxidizer (i.e. the gas must contain oxygen). As we all know from experience, combustion, or burnin ...
Answer
... Show that this reaction is spontaneous at 25 C. Using G° = H° - TS°, G° = (-198.4×103 J mol-1) – ((25+273) K) ×(-187.9 J mol-1) = -142400 J mol-1 = -142.4 kJ mol-1 As G° < 0, the reaction is spontaneous. If the volume of the reaction system is increased at 25 C, in which direction will the re ...
... Show that this reaction is spontaneous at 25 C. Using G° = H° - TS°, G° = (-198.4×103 J mol-1) – ((25+273) K) ×(-187.9 J mol-1) = -142400 J mol-1 = -142.4 kJ mol-1 As G° < 0, the reaction is spontaneous. If the volume of the reaction system is increased at 25 C, in which direction will the re ...
Balancing Chemical Equations Academic Success Center Science Tutoring Area *
... If polyatomic ions are the same on the reactant side as the product side, balance the polyatomic ion as one group or one ion ●2(NH4)3PO4(aq) + 3 CaCl2 Ca3(PO4)2(s) + 6 NH4Cl(aq) 24 hydrogens, 6 ...
... If polyatomic ions are the same on the reactant side as the product side, balance the polyatomic ion as one group or one ion ●2(NH4)3PO4(aq) + 3 CaCl2 Ca3(PO4)2(s) + 6 NH4Cl(aq) 24 hydrogens, 6 ...
Chemical reactions
... tabulation of standard enthalpies of formation is presented aside, including the chemical elements, for which the surname 'formation' added to the standard enthalpy is irrelevant: hf⊕= h⊕=h(T⊕,p⊕)=0. Notice that standard enthalpies of formation are usually negative because the formation reaction is ...
... tabulation of standard enthalpies of formation is presented aside, including the chemical elements, for which the surname 'formation' added to the standard enthalpy is irrelevant: hf⊕= h⊕=h(T⊕,p⊕)=0. Notice that standard enthalpies of formation are usually negative because the formation reaction is ...
Synthesis, characterization of some transition metal
... diffractogram of Ni(II) complex of L had twenty reflections with maxima at 2θ = 10.59° corresponding to d value 4.18Å. The diffractogram of Cu(II) complex of L had eighteen reflections with maxima at 2θ = 14.96° corresponding to d value 2.98Å.The diffractogram of Zn(II) complex of L shows twenty one ...
... diffractogram of Ni(II) complex of L had twenty reflections with maxima at 2θ = 10.59° corresponding to d value 4.18Å. The diffractogram of Cu(II) complex of L had eighteen reflections with maxima at 2θ = 14.96° corresponding to d value 2.98Å.The diffractogram of Zn(II) complex of L shows twenty one ...
File
... solution is added drop by drop to a warm solution Select the one lettered choice that best fits each of sodium hydroxide. statement and then blacken the corresponding space on 13. No precipitate is formed when a dilute solution of the answer sheet. A choice may be used once, more H2SO4 is added to a ...
... solution is added drop by drop to a warm solution Select the one lettered choice that best fits each of sodium hydroxide. statement and then blacken the corresponding space on 13. No precipitate is formed when a dilute solution of the answer sheet. A choice may be used once, more H2SO4 is added to a ...
Relation between the characteristic molecular volume and
... The characteristic molecular volume used in correlations is a simple and effective measure of molecular volumes. It can be calculated by an atom-additive scheme and correlates with van der Waals volumes of molecules. The united set of molecules includes 58 species that belong to different classes of ...
... The characteristic molecular volume used in correlations is a simple and effective measure of molecular volumes. It can be calculated by an atom-additive scheme and correlates with van der Waals volumes of molecules. The united set of molecules includes 58 species that belong to different classes of ...
Journal of Physical and Chemical Reference Data
... number of ways and it is therefore literally impossible to catalog all the possible heats of reaction. To get around this problem we define for each substance a standard reaction and tabulate its associated heat of reaction. These reactions and their associated heats of reaction can then be used to ...
... number of ways and it is therefore literally impossible to catalog all the possible heats of reaction. To get around this problem we define for each substance a standard reaction and tabulate its associated heat of reaction. These reactions and their associated heats of reaction can then be used to ...
14.1 Dynamic Equilibrium, Keq , and the Mass Action Expression
... When making assumptions, if a reaction has a relatively small keq and a relatively large initial reactant concentration, then the concentration change (x) can often be neglected without introducing significant error. This does not mean x = 0, because then this would mean there is no reaction. It mea ...
... When making assumptions, if a reaction has a relatively small keq and a relatively large initial reactant concentration, then the concentration change (x) can often be neglected without introducing significant error. This does not mean x = 0, because then this would mean there is no reaction. It mea ...
Chapter 8 - profpaz.com
... 2. What mass of carbon dioxide will be produced from the reaction of 175 g of propane, as shown above? 175 g C3H8 x x x = ...
... 2. What mass of carbon dioxide will be produced from the reaction of 175 g of propane, as shown above? 175 g C3H8 x x x = ...
Fundamental Equilibrium Concepts
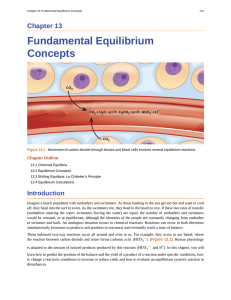
... The formation of NO2 from N2O4 is a reversible reaction, which is identified by the equilibrium arrow (⇌) . All reactions are reversible, but many reactions, for all practical purposes, proceed in one direction until the reactants are exhausted and will reverse only under certain conditions. Such re ...
... The formation of NO2 from N2O4 is a reversible reaction, which is identified by the equilibrium arrow (⇌) . All reactions are reversible, but many reactions, for all practical purposes, proceed in one direction until the reactants are exhausted and will reverse only under certain conditions. Such re ...