Date - PetyaPisanScienceAQ
... 3) Propose two additional experiments you could use to test the physical properties of the two metals. (2I) a) density of the 2 metals b) melting point c) electrical conductivity 4) Identify the reactants and the products (if any) for the reaction with each metal in step # 6 of the procedure. Write ...
... 3) Propose two additional experiments you could use to test the physical properties of the two metals. (2I) a) density of the 2 metals b) melting point c) electrical conductivity 4) Identify the reactants and the products (if any) for the reaction with each metal in step # 6 of the procedure. Write ...
ap chemistry 2005/2006
... Galvanic cells (Jan 30,31, Feb 1-3, Standard reduction potentials Feb 6-10, 13) Cell potential, electrical work, and free energy Cell potential and concentrations Batteries Corrosion Electrolysis Commercial electrolytic processes Lab: Electrochemical Cells –several different half-cells are prepared ...
... Galvanic cells (Jan 30,31, Feb 1-3, Standard reduction potentials Feb 6-10, 13) Cell potential, electrical work, and free energy Cell potential and concentrations Batteries Corrosion Electrolysis Commercial electrolytic processes Lab: Electrochemical Cells –several different half-cells are prepared ...
Thermochemistry Exam Review Questions
... an unknown solution. What is the pH for the unknown solution likely to be? A. 1.2 B. 3.0 C. 5.3 D. 9.0 12. What is the name of the ion when a positively charged proton combines with a water molecule? A. ammonium ion B. hydrogen ion C. hydronium ion D. hydroxide ion 13. What is the term for a substan ...
... an unknown solution. What is the pH for the unknown solution likely to be? A. 1.2 B. 3.0 C. 5.3 D. 9.0 12. What is the name of the ion when a positively charged proton combines with a water molecule? A. ammonium ion B. hydrogen ion C. hydronium ion D. hydroxide ion 13. What is the term for a substan ...
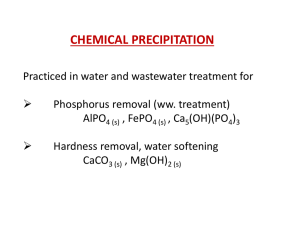
Phosphorus Removal from Wastewater by Chemical Precipitation
... However, these reactions are simple and must be considered in the light of many competing reactions. ...
... However, these reactions are simple and must be considered in the light of many competing reactions. ...
Chapter 2 Geochemical Reactions
... Since ancient times, cities and civilizations have been built around reliable sources of potable water. Constantinople, now Istanbul, has even today at its heart a deep cistern protected by surrounding ramparts and stone arches that supplied groundwater to its population. Jiao (2007) describes a 560 ...
... Since ancient times, cities and civilizations have been built around reliable sources of potable water. Constantinople, now Istanbul, has even today at its heart a deep cistern protected by surrounding ramparts and stone arches that supplied groundwater to its population. Jiao (2007) describes a 560 ...
Ksp - ChemConnections
... Calcium phosphate (Ca3(PO4)2 is only slightly soluble. In fact, it is common ingredient in phosphate rock and is a major source of phosphate fertilizer. If the molar solubility is 2 × 10–7, what is the value of the Ksp? A. 3 × 10–34 B. 2 × 10–10 C. 3 × 10–32 D. None of the above. ...
... Calcium phosphate (Ca3(PO4)2 is only slightly soluble. In fact, it is common ingredient in phosphate rock and is a major source of phosphate fertilizer. If the molar solubility is 2 × 10–7, what is the value of the Ksp? A. 3 × 10–34 B. 2 × 10–10 C. 3 × 10–32 D. None of the above. ...
AP Chemistry Syllabus - Old Mill High School
... equipment, and safety procedures Visualizing Concepts – Brown/LeMay Chapters 1 and 2 Chapter Exercises – Brown/LeMay Chapter 1 and 2 Labs: (85 minutes and 60 minutes out of class each lab) Unless otherwise noted all labs are hands-on Density Lab - Liquids, regular and irregular solids liquid t ...
... equipment, and safety procedures Visualizing Concepts – Brown/LeMay Chapters 1 and 2 Chapter Exercises – Brown/LeMay Chapter 1 and 2 Labs: (85 minutes and 60 minutes out of class each lab) Unless otherwise noted all labs are hands-on Density Lab - Liquids, regular and irregular solids liquid t ...
U-6 Stoichiometry Notes
... In chemistry we make some measurements by counting and other measurements by weighing, determining volume, etc. Which technique we employ is determined, in large part, by our purpose. It is also necessary, when determining which technique to use, to consider what type of measurement is easiest to ma ...
... In chemistry we make some measurements by counting and other measurements by weighing, determining volume, etc. Which technique we employ is determined, in large part, by our purpose. It is also necessary, when determining which technique to use, to consider what type of measurement is easiest to ma ...
Chemical properties of amines:
... The higher the value of Kb is, the greater the dissociation of the base. Amines have Kb values slightly higher than amonia. Thus aliphatic amines are stronger bases than amonia. Like amonia, compounds with amino groups can also neutralize hydronium ions. This neutralization occurs very rapidly and e ...
... The higher the value of Kb is, the greater the dissociation of the base. Amines have Kb values slightly higher than amonia. Thus aliphatic amines are stronger bases than amonia. Like amonia, compounds with amino groups can also neutralize hydronium ions. This neutralization occurs very rapidly and e ...
Determination of Active Ingredients in Commercial Bleach and Vinegar
... Part A: Analysis of commercial bleach solution 1. Pipet 10.0 mL of bleach (the density of liquid bleach is 1.084 g/mL) into a 100 mL volumetric flask and add about 50 mL of distilled water. Shake it well and fill it to the mark with distilled water. Mix thoroughly by inverting the stoppered volumetr ...
... Part A: Analysis of commercial bleach solution 1. Pipet 10.0 mL of bleach (the density of liquid bleach is 1.084 g/mL) into a 100 mL volumetric flask and add about 50 mL of distilled water. Shake it well and fill it to the mark with distilled water. Mix thoroughly by inverting the stoppered volumetr ...
Characterization of Multi-constituent Substances for REACH
... Analysis of the reaction mass without physical separation of its constituents There are several reasons why this approach may be adopted in the characterization process: Physical separation may be difficult or it may not be possible to provide unequivocal evidence to support the composition of the s ...
... Analysis of the reaction mass without physical separation of its constituents There are several reasons why this approach may be adopted in the characterization process: Physical separation may be difficult or it may not be possible to provide unequivocal evidence to support the composition of the s ...
Strong and weak acids and bases
... poor conductor with pH of 6: weak acid fast reaction with magnesium: strong acid [H+] = 10 -10M: weak base [H+] = 2.3 x 10 -4M: weak acid neutralized 0.1M HCl quickly: strong base good conductor with pH of 13: strong base slow reaction with calcium: weak acid has a high -ΔH when reacting with a base ...
... poor conductor with pH of 6: weak acid fast reaction with magnesium: strong acid [H+] = 10 -10M: weak base [H+] = 2.3 x 10 -4M: weak acid neutralized 0.1M HCl quickly: strong base good conductor with pH of 13: strong base slow reaction with calcium: weak acid has a high -ΔH when reacting with a base ...