Determination of the diffusion coefficient of sucrose in water and its
... k is the Boltzmann constant and T is the absolute temperature. There are several important assumptions implicit in equation (17). Two of these are that the solute is spherical, and considerably larger than the solvent molecules. Deviations from spherical geometry (such as oblate or prolate ellipsoid ...
... k is the Boltzmann constant and T is the absolute temperature. There are several important assumptions implicit in equation (17). Two of these are that the solute is spherical, and considerably larger than the solvent molecules. Deviations from spherical geometry (such as oblate or prolate ellipsoid ...
10. Factors Affecting the Rate of a Chemical Reaction
... exchange atoms) will have an effective (successful) collision that leads to the formation of products. There are four factors that can influence the rate of a chemical reaction: 1. The Ionic or Molecular Nature of the Reactants In general, chemical reactions that occur between ions in aqueous soluti ...
... exchange atoms) will have an effective (successful) collision that leads to the formation of products. There are four factors that can influence the rate of a chemical reaction: 1. The Ionic or Molecular Nature of the Reactants In general, chemical reactions that occur between ions in aqueous soluti ...
Experiment 1
... the heat of reaction or the enthalpy change. The symbol ΔH is used to denote the enthalpy change. If heat is evolved, the reaction is exothermic (ΔH 0); and if heat is absorbed, the reaction is endothermic (ΔH 0). In this experiment, you will calculate the enthalpy change of the above displaceme ...
... the heat of reaction or the enthalpy change. The symbol ΔH is used to denote the enthalpy change. If heat is evolved, the reaction is exothermic (ΔH 0); and if heat is absorbed, the reaction is endothermic (ΔH 0). In this experiment, you will calculate the enthalpy change of the above displaceme ...
JCE0198 p0087 A Kinetics Experiment To Demonstrate the Role of
... better leaving group than the OH{ and hence the reaction between I{ and H3O 2+ should be more favorable. They can also see that kcat is in fact equal to K1 kH and that the catalyzed mechanism need not be termolecular. The students repeat the same series of reactions at a low temperature to obtain va ...
... better leaving group than the OH{ and hence the reaction between I{ and H3O 2+ should be more favorable. They can also see that kcat is in fact equal to K1 kH and that the catalyzed mechanism need not be termolecular. The students repeat the same series of reactions at a low temperature to obtain va ...
Document
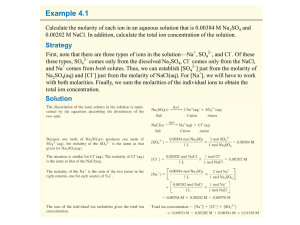
... Seawater is essentially 0.438 M NaCl and 0.0512 M MgCl 2, together with several other minor solutes. What are the molarities of Na+, Mg2+, and Cl– in seawater? ...
... Seawater is essentially 0.438 M NaCl and 0.0512 M MgCl 2, together with several other minor solutes. What are the molarities of Na+, Mg2+, and Cl– in seawater? ...
Rotational−Vibrational Levels of Diatomic Molecules Represented
... ) ω2e /4ωexe, is applied. However, the Birge-Sponer approximation can lead to errors in values of D that can be as large as 30%). In this work, we calculate β from the relationship 6 (Tables 1 and 2) because the constants ωe and D available in literature are usually more accurate than the anharmonic ...
... ) ω2e /4ωexe, is applied. However, the Birge-Sponer approximation can lead to errors in values of D that can be as large as 30%). In this work, we calculate β from the relationship 6 (Tables 1 and 2) because the constants ωe and D available in literature are usually more accurate than the anharmonic ...
AP CHEMISTRY MRS. SPENCER CHAPTER 4 TEST: SOLUTION
... included below only 1 of the 3 reactions listed in this question.) 4. For (each of) the following (three) reaction(s), in part (i) write a balanced equation for the reaction and in part (ii) answer the question about the reaction. In part (i), coefficients should be in terms of lowest whole numbers. ...
... included below only 1 of the 3 reactions listed in this question.) 4. For (each of) the following (three) reaction(s), in part (i) write a balanced equation for the reaction and in part (ii) answer the question about the reaction. In part (i), coefficients should be in terms of lowest whole numbers. ...
Effect of pH on the Electrodeposition of ZnTe Film from a Citric Acid
... the amounts of Zn2þ ions concentration in the bath, which are necessary to deposit Zn atom, are different. The effects of the difference in these reaction paths on the electrodeposited film’s composition and crystallization were then investigated. Figure 2 shows the XRD diffraction patterns of the deposi ...
... the amounts of Zn2þ ions concentration in the bath, which are necessary to deposit Zn atom, are different. The effects of the difference in these reaction paths on the electrodeposited film’s composition and crystallization were then investigated. Figure 2 shows the XRD diffraction patterns of the deposi ...
Chapter 16 Aqueous Ionic Equilibrium Lecture Presentation
... point and excess acid region. • The initial pH is determined using the Ka of the weak acid. • The pH in the excess acid region is determined as you would determine the pH of a buffer. • The pH at the equivalence point is determined using the Kb of the conjugate base of the weak acid. • The pH after ...
... point and excess acid region. • The initial pH is determined using the Ka of the weak acid. • The pH in the excess acid region is determined as you would determine the pH of a buffer. • The pH at the equivalence point is determined using the Kb of the conjugate base of the weak acid. • The pH after ...
im11
... 66. No, drinking only pure water with no detectable salt content would not be healthy for two main reasons. First, your body requires ions such as sodium, potassium, calcium, and magnesium for normal function. Many of the ions we need come from the water we drink. Second, water devoid of ions is ver ...
... 66. No, drinking only pure water with no detectable salt content would not be healthy for two main reasons. First, your body requires ions such as sodium, potassium, calcium, and magnesium for normal function. Many of the ions we need come from the water we drink. Second, water devoid of ions is ver ...
Chapter 4 Quantities of Reactants and Products 4.1 Chemical
... In a combustion analysis of a compound containing carbon and hydrogen, the compound reacts with oxygen and all of the carbon in the compound is converted to carbon dioxide and the hydrogen in the compound is converted to water. 2 C4H10(g) + 13 O2(g) ---> 8 CO2(g) + 10 H2O(g) The law of conservation ...
... In a combustion analysis of a compound containing carbon and hydrogen, the compound reacts with oxygen and all of the carbon in the compound is converted to carbon dioxide and the hydrogen in the compound is converted to water. 2 C4H10(g) + 13 O2(g) ---> 8 CO2(g) + 10 H2O(g) The law of conservation ...
Chemistry - CBSE Academic
... Crystallization of impure sample of any one of the following: Alum, Copper Sulphate, Benzoic Acid. ...
... Crystallization of impure sample of any one of the following: Alum, Copper Sulphate, Benzoic Acid. ...
chapter 15 acids and bases
... Step 4: Write the ionization constant expression in terms of the equilibrium concentrations. Knowing the value of the equilibrium constant (Ka), solve for x. You can look up the Ka value in Table 15.3 of the text. Ka = ...
... Step 4: Write the ionization constant expression in terms of the equilibrium concentrations. Knowing the value of the equilibrium constant (Ka), solve for x. You can look up the Ka value in Table 15.3 of the text. Ka = ...
KEY - Unit 10 - Practice Questions
... 40. According to Reference Table J, which of these metals will react most readily with 1.0 M HCl to produce H2(g)? (1) Ca (2) K (3) Mg (4) Zn 41. Under standard conditions, which metal will react with 0.1 M HCl to liberate hydrogen gas? (1) Ag (2) Au (3) Cu (4) Mg 42. Because tap water is slightly a ...
... 40. According to Reference Table J, which of these metals will react most readily with 1.0 M HCl to produce H2(g)? (1) Ca (2) K (3) Mg (4) Zn 41. Under standard conditions, which metal will react with 0.1 M HCl to liberate hydrogen gas? (1) Ag (2) Au (3) Cu (4) Mg 42. Because tap water is slightly a ...
The Process of Chemical Reactions
... Thus, as the reaction begins, an input of energy is necessary to produce the activated complex; as the reaction proceeds, and the system shifts from the activated complex to products, energy is released. In a chemical reaction, the minimum energy necessary for reaching the activated complex and proc ...
... Thus, as the reaction begins, an input of energy is necessary to produce the activated complex; as the reaction proceeds, and the system shifts from the activated complex to products, energy is released. In a chemical reaction, the minimum energy necessary for reaching the activated complex and proc ...
Reactions and Solutions - Louisiana Tech University
... occur in solution. A solution is composed of one or more solutes dissolved in a solvent. In aqueous solutions, the solvent is water. Liquid solutions are clear and transparent with no visible particles of solute. They may be colored or colorless, depending on the properties of the solute and solvent ...
... occur in solution. A solution is composed of one or more solutes dissolved in a solvent. In aqueous solutions, the solvent is water. Liquid solutions are clear and transparent with no visible particles of solute. They may be colored or colorless, depending on the properties of the solute and solvent ...
pdf version - Joliet Junior College
... Review: What follows is a recap of the most important topics covered in CHM 101. We will use this material throughout CHM 102, so please ensure that you are familiar with the following questions, as well as the Ch3 & 4 HWK questions, before we move on to the Ch 11 material. Top Tip: Committing to a ...
... Review: What follows is a recap of the most important topics covered in CHM 101. We will use this material throughout CHM 102, so please ensure that you are familiar with the following questions, as well as the Ch3 & 4 HWK questions, before we move on to the Ch 11 material. Top Tip: Committing to a ...
Chapter 15 Chemical Equilibrium
... • write Keq expression, in units of pressure or concentration • assess the Keq with regard to relative concentrations of reactants and products • manipulate chemical equations and Keq – reciprocal, multiplication, application of Hess’s Law ...
... • write Keq expression, in units of pressure or concentration • assess the Keq with regard to relative concentrations of reactants and products • manipulate chemical equations and Keq – reciprocal, multiplication, application of Hess’s Law ...