Free Energy Examples
... Suppose an isolated box of volume 2V is divided into two equal compartments. An ideal gas occupies half of the container and the other half is empty. When the partition separating the two halves of the box is removed and the system reaches equilibrium again, how does the new internal energy of the ...
... Suppose an isolated box of volume 2V is divided into two equal compartments. An ideal gas occupies half of the container and the other half is empty. When the partition separating the two halves of the box is removed and the system reaches equilibrium again, how does the new internal energy of the ...
Removing the Mystery of Entropy and Thermodynamics – Part III
... system, a larger system, and, finally, an ideal reservoir for which S is a linear function of the enthalpy H. Figure 3(b) shows a finite system with a concave spreading function initially in state A with temperature TA, the reciprocal of the slope. It then interacts thermally with an ideal reservoir ...
... system, a larger system, and, finally, an ideal reservoir for which S is a linear function of the enthalpy H. Figure 3(b) shows a finite system with a concave spreading function initially in state A with temperature TA, the reciprocal of the slope. It then interacts thermally with an ideal reservoir ...
EGU2016-10322 - CO Meeting Organizer
... Because of that, modelling the viscosity or the heat capacity of silicate melts is crucial in order to model the physical processes they are involved in. The Adam and Gibbs theory of viscous flow offers a thermodynamic framework that assumes that the viscosity η (Pa s) at a temperature T (K) of a me ...
... Because of that, modelling the viscosity or the heat capacity of silicate melts is crucial in order to model the physical processes they are involved in. The Adam and Gibbs theory of viscous flow offers a thermodynamic framework that assumes that the viscosity η (Pa s) at a temperature T (K) of a me ...
Entropy and The Second Law of Thermodynamics
... The total entropy of all systems taking part in a process never decreases. It remains the same only if the process is quasistatic. The entropy of an isolated (closed) system can never decrease. It remains the same only if all internal processes are quasistatic. Note that real processes are never exa ...
... The total entropy of all systems taking part in a process never decreases. It remains the same only if the process is quasistatic. The entropy of an isolated (closed) system can never decrease. It remains the same only if all internal processes are quasistatic. Note that real processes are never exa ...
Chemical Thermodynamics
... • So, the final state of a system can be more probable than its initial state in either or both of two ways: 1. Energy can be dispersed over a greater number and variety of molecules 2. The particles of the system can be more dispersed (more disordered) ...
... • So, the final state of a system can be more probable than its initial state in either or both of two ways: 1. Energy can be dispersed over a greater number and variety of molecules 2. The particles of the system can be more dispersed (more disordered) ...
Ch 20 Thermodynamics
... S, A measure of molecular randomness or disorder. Thermodynamic function that describes number of arrangements that are available to a system existing in a given state. Probability of occurrence of a particular arrangement(state) depends on the number of ways(microstates) in which it can be ar ...
... S, A measure of molecular randomness or disorder. Thermodynamic function that describes number of arrangements that are available to a system existing in a given state. Probability of occurrence of a particular arrangement(state) depends on the number of ways(microstates) in which it can be ar ...
Document
... Change in entropy of the surroundings: ΔSsur If we consider a transfer of heat dqsur to the surroundings, which can be assumed to be a reservoir of constant volume. The energy transferred can be identified with the change in internal energy dUsur is independent of how change brought about (U ...
... Change in entropy of the surroundings: ΔSsur If we consider a transfer of heat dqsur to the surroundings, which can be assumed to be a reservoir of constant volume. The energy transferred can be identified with the change in internal energy dUsur is independent of how change brought about (U ...
Entropy in chemical thermodynamics
... surroundings), and do not purport to measure the "useful" energy. When heat is added to a system at high temperature, the increase in entropy is small. When heat is added to a system at low temperature, the increase in entropy is great. This can be quantified as follows: in thermal systems, changes ...
... surroundings), and do not purport to measure the "useful" energy. When heat is added to a system at high temperature, the increase in entropy is small. When heat is added to a system at low temperature, the increase in entropy is great. This can be quantified as follows: in thermal systems, changes ...
Laws of Thermodynamics
... conserved. However, there was a second class of suggested perpetual motion machines which didn’t work but which were consistent with energy conservation. Another law of thermodynamics was introduced to summarize the fact that this class of perpetual motion machines also does not work. This is the se ...
... conserved. However, there was a second class of suggested perpetual motion machines which didn’t work but which were consistent with energy conservation. Another law of thermodynamics was introduced to summarize the fact that this class of perpetual motion machines also does not work. This is the se ...
Document
... Change in entropy of the surroundings: ΔSsur If we consider a transfer of heat dqsur to the surroundings, which can be assumed to be a reservoir of constant volume. The energy transferred can be identified with the change in internal energy dUsur is independent of how change brought about (U ...
... Change in entropy of the surroundings: ΔSsur If we consider a transfer of heat dqsur to the surroundings, which can be assumed to be a reservoir of constant volume. The energy transferred can be identified with the change in internal energy dUsur is independent of how change brought about (U ...
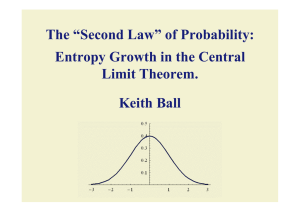
The “Second Law” of Probability: Entropy Growth in the Central Limit
... It costs k questions to identify a state from among 2k equally likely states. It costs log2 n questions to identify a state from among n equally likely states: to identify a state with probability 1/n. ...
... It costs k questions to identify a state from among 2k equally likely states. It costs log2 n questions to identify a state from among n equally likely states: to identify a state with probability 1/n. ...
Lecture 4
... “cubic” lattice in 3N dimensional momentum space with cube edge h/L and so the volume of phase space per state is (h/L)3N L3N = h3N . The factor of N ! arises because from quantum mechanics we recognize that identical particles are indistinguishable. In a two-particle, one-dimensional system, for ex ...
... “cubic” lattice in 3N dimensional momentum space with cube edge h/L and so the volume of phase space per state is (h/L)3N L3N = h3N . The factor of N ! arises because from quantum mechanics we recognize that identical particles are indistinguishable. In a two-particle, one-dimensional system, for ex ...
Chapter Summary
... A cycle is a sequence of processes that returns a system to its original state. The cycle as a whole satisfies the first law of thermodynamics, as does each of its processes. The change in internal energy for any cycle is always zero, because the system returns to its initial state, and the area of ...
... A cycle is a sequence of processes that returns a system to its original state. The cycle as a whole satisfies the first law of thermodynamics, as does each of its processes. The change in internal energy for any cycle is always zero, because the system returns to its initial state, and the area of ...
Entropy
... Depending on the topic and the context in which it is being used, the term entropy has been used to describe any of numerous phenomena. The word entropy was introduced in 1865 by Rudolf Clausius, a German physicist. Two main areas, thermodynamic entropy (including statistical mechanics) and informat ...
... Depending on the topic and the context in which it is being used, the term entropy has been used to describe any of numerous phenomena. The word entropy was introduced in 1865 by Rudolf Clausius, a German physicist. Two main areas, thermodynamic entropy (including statistical mechanics) and informat ...
Entropy
... • Consider two scenarios: – An ideal gas reversibly and adiabatically expands from volume Vi to Vf . ∗ The change in entropy is zero, dS = n0, because dQrev = 0. – An ideal gas freely and adiabatically expands from volume Vi to Vf . ∗ No work is done in a free expansion. ∗ Since this is adiabatic, a ...
... • Consider two scenarios: – An ideal gas reversibly and adiabatically expands from volume Vi to Vf . ∗ The change in entropy is zero, dS = n0, because dQrev = 0. – An ideal gas freely and adiabatically expands from volume Vi to Vf . ∗ No work is done in a free expansion. ∗ Since this is adiabatic, a ...
thermodynamics - La Salle High School
... If the entropy of each element in its most state is taken as zero at the absolute zero of temperature, every substance has a positive entropy. But at 0K, the entropy of substance may equals to 0, and does become zero in perfect crystalline solids. Implication: all perfect materials have the same ent ...
... If the entropy of each element in its most state is taken as zero at the absolute zero of temperature, every substance has a positive entropy. But at 0K, the entropy of substance may equals to 0, and does become zero in perfect crystalline solids. Implication: all perfect materials have the same ent ...