Document
... You have two styrofoam containers of water. Each holds 1 kg of water. In one the water temperature is 17°C, while in the other it is 37°C. The colder water is then poured into the warmer water, and the system is allowed to come to equilibrium. Is this process reversible or irreversible? ...
... You have two styrofoam containers of water. Each holds 1 kg of water. In one the water temperature is 17°C, while in the other it is 37°C. The colder water is then poured into the warmer water, and the system is allowed to come to equilibrium. Is this process reversible or irreversible? ...
Course name Thermodynamics Course code ENG.I.011 Department
... The objective of the course is to give the idea about the thermodynamic properties such as temperature, pressure, internal energy ,enthalpy, entropy etc. It also deals with the zeroth, first and second Law of Thermodynamics and their applications in environmental engineering field. Thermodynamics he ...
... The objective of the course is to give the idea about the thermodynamic properties such as temperature, pressure, internal energy ,enthalpy, entropy etc. It also deals with the zeroth, first and second Law of Thermodynamics and their applications in environmental engineering field. Thermodynamics he ...
HEALTH, AGEING AND ENTROPY
... be thought of as a measure of the degree of energy degradation in any process. The second law of thermodynamics is sometimes called the law of entropy. Clausius defined entropy mathematically as follows: If ΔQ is a small reversible change in the heat content of a body, and if the change takes place ...
... be thought of as a measure of the degree of energy degradation in any process. The second law of thermodynamics is sometimes called the law of entropy. Clausius defined entropy mathematically as follows: If ΔQ is a small reversible change in the heat content of a body, and if the change takes place ...
Defects - Script
... particles/cm3 or mol/cm 3 - you must know what is meant from the context. You are also supposed to know that if Boltzmanns constant k comes up in an equation, we are working with properties per particle, whereas the gas constant R signifies properties per mol. This, admittedly, is dangerous. But mul ...
... particles/cm3 or mol/cm 3 - you must know what is meant from the context. You are also supposed to know that if Boltzmanns constant k comes up in an equation, we are working with properties per particle, whereas the gas constant R signifies properties per mol. This, admittedly, is dangerous. But mul ...
Nonextensivity-Nonintensivity
... In order to explain the nature of nonextensivity of nanoscale systems the following discussion is presented: In thermodynamics, properties (variables) are classified as being either extensive or intensive. When properties of a system are independent of the number of particles present in the system, ...
... In order to explain the nature of nonextensivity of nanoscale systems the following discussion is presented: In thermodynamics, properties (variables) are classified as being either extensive or intensive. When properties of a system are independent of the number of particles present in the system, ...
Chap-4
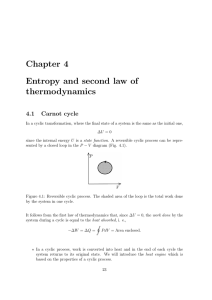
... 2. Remember that in a reversible process the deviation from equilibrium is infinitesimal. [Refer to the work of expansion problem considered previously in Section 3.6.] 3. In a reversible process, the entropy of the universe (i.e., the system plus surroundings) remains constant. We can examine rever ...
... 2. Remember that in a reversible process the deviation from equilibrium is infinitesimal. [Refer to the work of expansion problem considered previously in Section 3.6.] 3. In a reversible process, the entropy of the universe (i.e., the system plus surroundings) remains constant. We can examine rever ...
Entropy, free energy and equilibrium
... Matter disperses – gas fills a container, two liquids mix Heat disperses – hot object cools on cold surface Motion disperses – a ball stops bouncing The reverses of these three well known processes never occur spontaneously ...
... Matter disperses – gas fills a container, two liquids mix Heat disperses – hot object cools on cold surface Motion disperses – a ball stops bouncing The reverses of these three well known processes never occur spontaneously ...
Entropy and Free Energy
... State functions are properties that are determined by the state of the system, regardless of how that condition was achieved. energy, enthalpy, pressure, volume, temperature, entropy. ...
... State functions are properties that are determined by the state of the system, regardless of how that condition was achieved. energy, enthalpy, pressure, volume, temperature, entropy. ...
$doc.title
... Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure. Enthalpy is a thermodyn ...
... Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure. Enthalpy is a thermodyn ...
Thermodynamics - StrikerPhysics
... • Thermo (heat) dynamics (transfer) • Thermodynamic systems describe many many particles (molecules) which obey Newton’s laws for dynamics but which would be difficult to analyze due to their numbers. • We use macroscopic means for analysis of these systems of many particles - involving quantities s ...
... • Thermo (heat) dynamics (transfer) • Thermodynamic systems describe many many particles (molecules) which obey Newton’s laws for dynamics but which would be difficult to analyze due to their numbers. • We use macroscopic means for analysis of these systems of many particles - involving quantities s ...
Second Law of Thermodynamics
... infinitesimal amounts, and infinitesimally slowly, between equilibrium states such that the direction of the process can be reversed at any time. Remember that in a reversible process the deviation from equilibrium is infinitesimal. [Refer to the work of expansion problem considered previously in Se ...
... infinitesimal amounts, and infinitesimally slowly, between equilibrium states such that the direction of the process can be reversed at any time. Remember that in a reversible process the deviation from equilibrium is infinitesimal. [Refer to the work of expansion problem considered previously in Se ...
CHAP4
... infinitesimal amounts, and infinitesimally slowly, between equilibrium states such that the direction of the process can be reversed at any time. Remember that in a reversible process the deviation from equilibrium is infinitesimal. [Refer to the work of expansion problem considered previously in Se ...
... infinitesimal amounts, and infinitesimally slowly, between equilibrium states such that the direction of the process can be reversed at any time. Remember that in a reversible process the deviation from equilibrium is infinitesimal. [Refer to the work of expansion problem considered previously in Se ...
+ p
... Maxwell and Boltzmann developed a statistical model of thermodynamics in which the entropy appeared as a measure of “uncertainty”. Uncertainty should be interpreted as “uniformity” or “lack of differentiation”. ...
... Maxwell and Boltzmann developed a statistical model of thermodynamics in which the entropy appeared as a measure of “uncertainty”. Uncertainty should be interpreted as “uniformity” or “lack of differentiation”. ...