The “Second Law” of Probability: Entropy Growth in the Central Limit
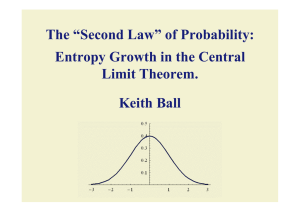
... Shannon-Stam shows that it increases as n goes from 1 to 2 (hence 2 to 4 and so on). Carlen and Soffer found uniform estimates for entropy jump from 1 to 2. It wasn’t known that entropy increases from 2 to 3. The difficulty is that you can't express the sum of 3 independent random variables in terms ...
... Shannon-Stam shows that it increases as n goes from 1 to 2 (hence 2 to 4 and so on). Carlen and Soffer found uniform estimates for entropy jump from 1 to 2. It wasn’t known that entropy increases from 2 to 3. The difficulty is that you can't express the sum of 3 independent random variables in terms ...
Entropy, a statistical approach
... Properties of entropy o For any given macroscopic state, there is a fixed number of microscopic states that could apply. Thus, the value for the entropy of a given macroscopic state is fixed. As such, entropy is a state function. It depends only on the macroscopic state of the system. o The change i ...
... Properties of entropy o For any given macroscopic state, there is a fixed number of microscopic states that could apply. Thus, the value for the entropy of a given macroscopic state is fixed. As such, entropy is a state function. It depends only on the macroscopic state of the system. o The change i ...
Practice Exam
... valve is opened, and steam flows through a turbine and into an evacuated tank (volume = 10 ft3). When the pressure in the tank equals the supply line pressure the valve is closed. The work output of the turbine during this process is 85 Btu/lbm of steam. Assuming the process to be adiabatic, find: ( ...
... valve is opened, and steam flows through a turbine and into an evacuated tank (volume = 10 ft3). When the pressure in the tank equals the supply line pressure the valve is closed. The work output of the turbine during this process is 85 Btu/lbm of steam. Assuming the process to be adiabatic, find: ( ...
As a system asymptotically approaches absolute zero of
... “The direction of spontaneous change as a result of a random influence is from order toward disorder.” “high-grade energy is dissipated irreversibly to a lowgrade form in every energy transformation.” Book on energy needs of the world, no religious slant. Energy, Science and the pursuit of susta ...
... “The direction of spontaneous change as a result of a random influence is from order toward disorder.” “high-grade energy is dissipated irreversibly to a lowgrade form in every energy transformation.” Book on energy needs of the world, no religious slant. Energy, Science and the pursuit of susta ...