9. Entropy 2nd and 3rd laws/ Thermodynamic processes / Droplet
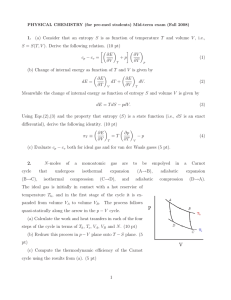
... (4pts) 2. Consider a “thermodynamic system” of two dices and let the energy of a certain throw (state of the system) be the sum of the two values of the dices. Calculate the respective entropy for all possible states (1), (2), (3),. . . . . . .,(11),(12). (3pts) 3. Both in words and equations. Descr ...
... (4pts) 2. Consider a “thermodynamic system” of two dices and let the energy of a certain throw (state of the system) be the sum of the two values of the dices. Calculate the respective entropy for all possible states (1), (2), (3),. . . . . . .,(11),(12). (3pts) 3. Both in words and equations. Descr ...