here
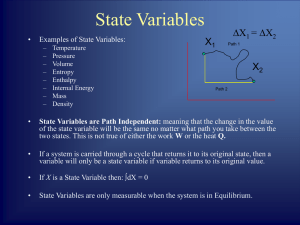
... Variables do not change with time. – Thermal Equilibrium No Temperature or Pressure Gradients in the System. – Mechanical Equilibrium No Unbalanced Forces or Torques in the System. – Chemical Equilibrium No tendency of the System to undergo Chemical ...
... Variables do not change with time. – Thermal Equilibrium No Temperature or Pressure Gradients in the System. – Mechanical Equilibrium No Unbalanced Forces or Torques in the System. – Chemical Equilibrium No tendency of the System to undergo Chemical ...
Sample Midterm - Ohio State Computer Science and Engineering
... Find the optimal cutpoint (split into two intervals) for AttrI using entropy as your basis for discretization. ...
... Find the optimal cutpoint (split into two intervals) for AttrI using entropy as your basis for discretization. ...
Document
... Change in entropy of the surroundings: ΔSsur If we consider a transfer of heat dqsur to the surroundings, which can be assumed to be a reservoir of constant volume. The energy transferred can be identified with the change in internal energy dUsur is independent of how change brought about (U ...
... Change in entropy of the surroundings: ΔSsur If we consider a transfer of heat dqsur to the surroundings, which can be assumed to be a reservoir of constant volume. The energy transferred can be identified with the change in internal energy dUsur is independent of how change brought about (U ...
CHAP4
... following (also assuming isobaric conditions): a) the change in internal energy b) the change in enthalpy c) the change in entropy 2. Calculate the change in entropy of 2 g of ice initially at -10 C which is converted to steam at 100 C due to heating. [ans: 17.3 J K-1] 3. A 200 g sample of dry air ...
... following (also assuming isobaric conditions): a) the change in internal energy b) the change in enthalpy c) the change in entropy 2. Calculate the change in entropy of 2 g of ice initially at -10 C which is converted to steam at 100 C due to heating. [ans: 17.3 J K-1] 3. A 200 g sample of dry air ...
thermodynamics - La Salle High School
... Heat flows from a high temperature reservoir to a low temperature body. The heat can be utilized to generate work. e.g. steam engine. ...
... Heat flows from a high temperature reservoir to a low temperature body. The heat can be utilized to generate work. e.g. steam engine. ...