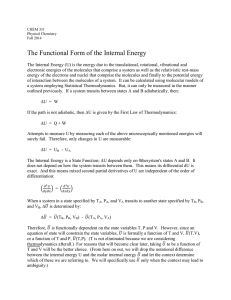
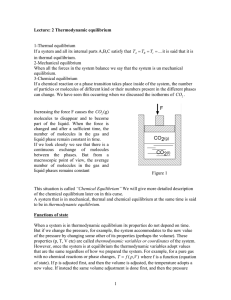
Temperature
... • Assume an object has an initial length L • That length increases by ΔL as the temperature changes by ΔT • We define the coefficient of linear expansion as ...
... • Assume an object has an initial length L • That length increases by ΔL as the temperature changes by ΔT • We define the coefficient of linear expansion as ...
Document
... • Is the reaction pictured exothermic or endothermic? • ENDOTHERMIC • Provide one justification for your answer to the previous question. • TEMPERATURE DECREASCED • Where was the energy absorbed from? • THE WATER • Would you predict that the products have a higher or lower energy than the reactants? ...
... • Is the reaction pictured exothermic or endothermic? • ENDOTHERMIC • Provide one justification for your answer to the previous question. • TEMPERATURE DECREASCED • Where was the energy absorbed from? • THE WATER • Would you predict that the products have a higher or lower energy than the reactants? ...
Chapter 1 Thermodynamics
... There are two standard ways to study the large N limit: • phenomenological (e.g. thermodynamics) and • fundamental (e.g. statistical mechanics). ...
... There are two standard ways to study the large N limit: • phenomenological (e.g. thermodynamics) and • fundamental (e.g. statistical mechanics). ...
Γ = Γ ∙ (1)
... 2oF/1000 ft, leading to net cooling of around 3.50F/1000 ft. Equation (1) can be solved for every temperature that appears on a thermodynamic chart, resulting in a family of lines called moist adiabats. Since the moist adiabatic rate given by (1) is not constant, these lines are curves whose mean sl ...
... 2oF/1000 ft, leading to net cooling of around 3.50F/1000 ft. Equation (1) can be solved for every temperature that appears on a thermodynamic chart, resulting in a family of lines called moist adiabats. Since the moist adiabatic rate given by (1) is not constant, these lines are curves whose mean sl ...
Quiz_MATH.rtf
... D) decreases at high temperature, increases at low E) stays the same 13. (C) Two monatomic ideal gases are in thermal equilibrium with each other. Gas A is composed of molecules with mass m while gas B is composed of molecules with mass 4m. The ratio of the average molecular kinetic energy KA/KB is ...
... D) decreases at high temperature, increases at low E) stays the same 13. (C) Two monatomic ideal gases are in thermal equilibrium with each other. Gas A is composed of molecules with mass m while gas B is composed of molecules with mass 4m. The ratio of the average molecular kinetic energy KA/KB is ...