0.1 Minimum Principles and Thermodynamic Potentials
... κT < 0. Then, ∂V |T = −P implies that A(V ) = − P dV , where the integration takes place along an isotherm. In the range (V1 , V2 ), Avdw (V ) has downward curvature. Now consider the point at Vm = V1 + V2 . We have that Avdw (Vm ) > Avdw (V1 )/2 + Avdw (V2 )/2 = Avdw (V1 /2) + Avdw (V2 /2). (The la ...
... κT < 0. Then, ∂V |T = −P implies that A(V ) = − P dV , where the integration takes place along an isotherm. In the range (V1 , V2 ), Avdw (V ) has downward curvature. Now consider the point at Vm = V1 + V2 . We have that Avdw (Vm ) > Avdw (V1 )/2 + Avdw (V2 )/2 = Avdw (V1 /2) + Avdw (V2 /2). (The la ...
Classical Physics
... - study of Thermal Energy of systems Temperature: a measure of thermal energy, units of Kelvins Room Temp ~ 290 K Temperature of an object is measured by the change in some physical property. Measuring device is called a thermometer. ...
... - study of Thermal Energy of systems Temperature: a measure of thermal energy, units of Kelvins Room Temp ~ 290 K Temperature of an object is measured by the change in some physical property. Measuring device is called a thermometer. ...
Corporate Profile
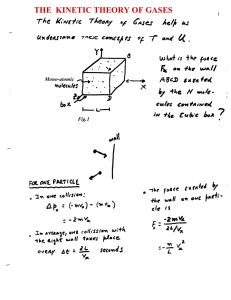
... Want to relate variables of state to each other. Experiments have found that gases follow approximately the same equation of state over a wide range of conditions. Although the atmosphere is a mixture of gases, it behaves as though it is a single “ideal” gas: • made up of a large number of molecules ...
... Want to relate variables of state to each other. Experiments have found that gases follow approximately the same equation of state over a wide range of conditions. Although the atmosphere is a mixture of gases, it behaves as though it is a single “ideal” gas: • made up of a large number of molecules ...
v = Y
... reservoir when heat is discarded into it (TC). ◦ Any finite temperature drop would result in an irreversible processes. ◦ Every process that involves heat transfer must be isothermal. ◦ Any process in which the the working substance is between TH and TC, there must be no heat transfer into the hot o ...
... reservoir when heat is discarded into it (TC). ◦ Any finite temperature drop would result in an irreversible processes. ◦ Every process that involves heat transfer must be isothermal. ◦ Any process in which the the working substance is between TH and TC, there must be no heat transfer into the hot o ...
GRE-thermo
... (C) The combined entropy of the gas and surroundings remains constant during each of the three processes. (D) For the complete cycle, the combined entropy of the gas and surroundings increases. (E) For the complete cycle, the entropy of the gas increases. 28. Which of the curves in the graph on the ...
... (C) The combined entropy of the gas and surroundings remains constant during each of the three processes. (D) For the complete cycle, the combined entropy of the gas and surroundings increases. (E) For the complete cycle, the entropy of the gas increases. 28. Which of the curves in the graph on the ...
t 0 - PhysicsEducation.net
... either into or out of the system. (Your answer to #7 should have decided whether it’s into or out of, or if instead x is really just 0 joules. Which is it?) Experiment B. Suppose that again we have 2 liters of each reactant solution. Now, though, let’s suppose that the concentration of each solution ...
... either into or out of the system. (Your answer to #7 should have decided whether it’s into or out of, or if instead x is really just 0 joules. Which is it?) Experiment B. Suppose that again we have 2 liters of each reactant solution. Now, though, let’s suppose that the concentration of each solution ...