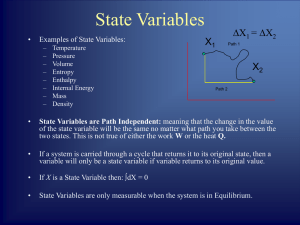
Problem set #2: 5
... diaphragm ruptures. Use the van der Waals equation for any nonideal behavior. Answer the following questions: (a) What is cv at the initial state? (b) Do you expect the temperature to increase, decrease, or remain constant. Justify your answer with molecular arguments. Be specific about the nature o ...
... diaphragm ruptures. Use the van der Waals equation for any nonideal behavior. Answer the following questions: (a) What is cv at the initial state? (b) Do you expect the temperature to increase, decrease, or remain constant. Justify your answer with molecular arguments. Be specific about the nature o ...
Document
... Pour a liter of water at 40 degrees C into a liter of water at 20 degrees C and the final temperature of the two becomes A) less than 30 degrees C. B) at or about 30 degrees C. C) more than 30 degrees C. ...
... Pour a liter of water at 40 degrees C into a liter of water at 20 degrees C and the final temperature of the two becomes A) less than 30 degrees C. B) at or about 30 degrees C. C) more than 30 degrees C. ...
calculating specific heat capacity - Mikus
... When in contact with each other, objects at different temperatures transfer thermal energy until they reach the same temperature. This is called thermal equilibrium. Conservation of energy requires that the thermal energy lost by the hotter object as it cools be equal to the thermal energy gained by ...
... When in contact with each other, objects at different temperatures transfer thermal energy until they reach the same temperature. This is called thermal equilibrium. Conservation of energy requires that the thermal energy lost by the hotter object as it cools be equal to the thermal energy gained by ...
HEAT- Chapter 9
... In general, the volume of a liquid will decrease as temperature decreases; the exception is water Solids tend to have the smallest coefficient of volume Coefficient of Volume Expansion- a number assigned to different material to show the thermal expansion characteristic of the material Gases ...
... In general, the volume of a liquid will decrease as temperature decreases; the exception is water Solids tend to have the smallest coefficient of volume Coefficient of Volume Expansion- a number assigned to different material to show the thermal expansion characteristic of the material Gases ...
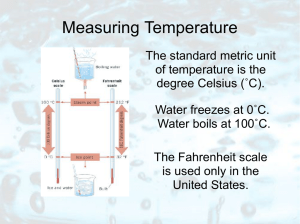
Temperature
... The heat transferred is proportional to the mass of the object, the specific heat capacity of the object and the temperature change the object undergoes. ...
... The heat transferred is proportional to the mass of the object, the specific heat capacity of the object and the temperature change the object undergoes. ...