2 Equations of Motion
... Now is the moment where many of you learn that you have taken the ideal gas law for granted all of these years. It may not come as a surprise that seawater is not an ideal gas. However, what may be surprising is that no simple expression like the ideal gas law exists for sea water, or liquids in gen ...
... Now is the moment where many of you learn that you have taken the ideal gas law for granted all of these years. It may not come as a surprise that seawater is not an ideal gas. However, what may be surprising is that no simple expression like the ideal gas law exists for sea water, or liquids in gen ...
Chapter 12: Engineering Thermodynamics
... scale. The Kelvin scale, a thermodynamic scale, can be elicited from the second law of thermodynamics. The definition of temperature following from the second law is valid over all temperature ranges and provides an essential connection between the several empirical measures of temperature. In parti ...
... scale. The Kelvin scale, a thermodynamic scale, can be elicited from the second law of thermodynamics. The definition of temperature following from the second law is valid over all temperature ranges and provides an essential connection between the several empirical measures of temperature. In parti ...
2.1 Introduction 2.2 Literature Review
... experiment may be regarded as a radiometric determination of the gold- point based on the theoretical value for σ, the result then being TAu = 133727± 0.40 K. This value is 0.31 K below the IPTS-68 value, derived by gas thermometry. Wray E.M [5] describes that tungsten bulb is used in a simple ammet ...
... experiment may be regarded as a radiometric determination of the gold- point based on the theoretical value for σ, the result then being TAu = 133727± 0.40 K. This value is 0.31 K below the IPTS-68 value, derived by gas thermometry. Wray E.M [5] describes that tungsten bulb is used in a simple ammet ...
Heat Capacity. Enthalpy. Magnetic Systems.
... isobaric process, where W ? is any other non-compressional work being done on the system. In general the enthalpy changes by ∆H = ∆U + ∆(P V ). But since the process under consideration is isobaric we have ∆H = ∆U + P ∆V = ∆Q + W ? , where we used the first law to get the last equality. You can see ...
... isobaric process, where W ? is any other non-compressional work being done on the system. In general the enthalpy changes by ∆H = ∆U + ∆(P V ). But since the process under consideration is isobaric we have ∆H = ∆U + P ∆V = ∆Q + W ? , where we used the first law to get the last equality. You can see ...
Session 12 : Monoprop ellant Thrusters
... Given some pressure p and an initial guess for the temperature T , we find y and x. At this point we need to verify that the temperature used in this calculation is consistent with the energy balance in Eq. (4). In all likelihood the temperature from Eq. (4) will not coincide with the initial guess, ...
... Given some pressure p and an initial guess for the temperature T , we find y and x. At this point we need to verify that the temperature used in this calculation is consistent with the energy balance in Eq. (4). In all likelihood the temperature from Eq. (4) will not coincide with the initial guess, ...
kauzmann temperature and the glass transition
... Joint Laboratory of Solid State Chemistry of Institute of Macromolecular Chemisty of Czech Academy of Sciences and University Pardubice, Studentská 84, Pardubice, Czech Republic Kauzmann temperature was investigated on the As2Se3 and As2S3 bulk glasses. Novel StepScan DSC technique was used to separ ...
... Joint Laboratory of Solid State Chemistry of Institute of Macromolecular Chemisty of Czech Academy of Sciences and University Pardubice, Studentská 84, Pardubice, Czech Republic Kauzmann temperature was investigated on the As2Se3 and As2S3 bulk glasses. Novel StepScan DSC technique was used to separ ...
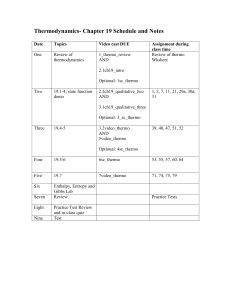
Schedule and sample problems
... occur in the value of ∆G˚ for this reaction as the temperature is increased? Explain your reasoning using thermodynamic principles. (c) What change, if any, would occur in the value of the equilibrium constant, Keq, for the situation described in (b)? Explain your reasoning. (d) The absolute tempera ...
... occur in the value of ∆G˚ for this reaction as the temperature is increased? Explain your reasoning using thermodynamic principles. (c) What change, if any, would occur in the value of the equilibrium constant, Keq, for the situation described in (b)? Explain your reasoning. (d) The absolute tempera ...
MEASURING SOIL RESISTIVITY
... At constant temperature, the resistivity of a metal conductor of a given length and section is a specific characteristic of the material and depends on its nature. It is expressed in ohm-meters (Ω.m): ρ = R x S/L where ρ = resistivity of the material (Ω.m); R = resistance measured (Ω); L = length of ...
... At constant temperature, the resistivity of a metal conductor of a given length and section is a specific characteristic of the material and depends on its nature. It is expressed in ohm-meters (Ω.m): ρ = R x S/L where ρ = resistivity of the material (Ω.m); R = resistance measured (Ω); L = length of ...