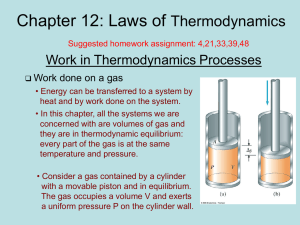
Chapter 1
... Variable Flow Process: In some flow process, mass flow rate is not steady but varies with respect to time. In such a case, the difference in energy flow is stored in system as ∆Ev. ...
... Variable Flow Process: In some flow process, mass flow rate is not steady but varies with respect to time. In such a case, the difference in energy flow is stored in system as ∆Ev. ...
Influence of the ambient temperature during heat pipe
... during filling and exhausting heat pipes. This assumption is confirmed by experimental measurements which were used in three different working materials at three different operating temperatures. As working materials were used water, ethanol and Fluorinert FC72. As the most widely used working fluid ...
... during filling and exhausting heat pipes. This assumption is confirmed by experimental measurements which were used in three different working materials at three different operating temperatures. As working materials were used water, ethanol and Fluorinert FC72. As the most widely used working fluid ...
Heat Capacity - Uplift North Hills Prep
... Q. Explain why supply of latent heat causes a change in potential energy but not kinetic energy. A. Kelvin temperature is proportional to the KE of the molecules. Thus if the temperature hasn’t increased then the KE has also not increased. Work has been done against forces, changing the position of ...
... Q. Explain why supply of latent heat causes a change in potential energy but not kinetic energy. A. Kelvin temperature is proportional to the KE of the molecules. Thus if the temperature hasn’t increased then the KE has also not increased. Work has been done against forces, changing the position of ...
Sample pages 2 PDF
... The system is a separate part of the world (Fig. 2.3a). Everything that does not belong to the system is called the surroundings. The system is separated from the surroundings by walls. These concepts are present in discussions of all phenomena described by thermodynamics. The wall is called adiabat ...
... The system is a separate part of the world (Fig. 2.3a). Everything that does not belong to the system is called the surroundings. The system is separated from the surroundings by walls. These concepts are present in discussions of all phenomena described by thermodynamics. The wall is called adiabat ...
Specific heat, magnetic susceptibility, resistivity and thermal
... The resistivity (Fig. 1a) was measured in a DC four-lead technique with offset compensation in a Quantum Design MPMS-5 magnetometer using a universal probe, a DC current source and a DC nanovoltmeter. The resistance of the contacts realized with silver paint were of the order of one Ohm each. Data ...
... The resistivity (Fig. 1a) was measured in a DC four-lead technique with offset compensation in a Quantum Design MPMS-5 magnetometer using a universal probe, a DC current source and a DC nanovoltmeter. The resistance of the contacts realized with silver paint were of the order of one Ohm each. Data ...
Thermochemistry
... (usually) and is the amt of substance ∆T [=] C° and is the change in temperature ∆T also equal Tf - Ti where Tf and Ti represent the final and initial temperatures Note: Q will be positive if the temp is increasing (∆T +) and negative if the temp is decreasing (∆T -). Cp is the amount of heat needed ...
... (usually) and is the amt of substance ∆T [=] C° and is the change in temperature ∆T also equal Tf - Ti where Tf and Ti represent the final and initial temperatures Note: Q will be positive if the temp is increasing (∆T +) and negative if the temp is decreasing (∆T -). Cp is the amount of heat needed ...
Expt. 5: Binary Phase Diagram CHEM 366 V-1 Binary Solid
... may behave more or less independent of each other but merely diluted, i.e., an ideal solution or mixture, or there may be substantial chemical interaction or complex formation between the constituents. The study of such mixtures can lead to an understanding of the most fundamental intermolecular int ...
... may behave more or less independent of each other but merely diluted, i.e., an ideal solution or mixture, or there may be substantial chemical interaction or complex formation between the constituents. The study of such mixtures can lead to an understanding of the most fundamental intermolecular int ...