Statistical Physics
... 4) An ideal gas ( =1.4) expands quasi-statically and adiabatically. If the final temperature is one third the initial temperature so by what factor does its volume change? a. 10 c. 16 b. 20 d. 12 5) Following question 4 above, by what factor does its pressure change? a. 1 c. 0.02 b. 1.2 d. 2 6) O ...
... 4) An ideal gas ( =1.4) expands quasi-statically and adiabatically. If the final temperature is one third the initial temperature so by what factor does its volume change? a. 10 c. 16 b. 20 d. 12 5) Following question 4 above, by what factor does its pressure change? a. 1 c. 0.02 b. 1.2 d. 2 6) O ...
Sensing Properties of a Novel Temperature Sensor Based on Field
... non-self-sustaining discharge. The sharp tips of nanotubes generate high electric fields at relatively low voltages, lowering the work function of electrons emitted by CNTs, and thereby enabling the safe operation of such sensors. Due to the temperature effect on the electron emission of CNTs, the c ...
... non-self-sustaining discharge. The sharp tips of nanotubes generate high electric fields at relatively low voltages, lowering the work function of electrons emitted by CNTs, and thereby enabling the safe operation of such sensors. Due to the temperature effect on the electron emission of CNTs, the c ...
14 Enthalpy of neutralization
... In this lab a similar experiment will be performed to find the enthalpy change (H) that occurs during a neutralization reaction between sodium hydroxide and one of three acids (HA) that will be assigned to you. HnA + nNaOH NanA + nH2O The calorimeter will be a “coffee cup” calorimeter, using two ...
... In this lab a similar experiment will be performed to find the enthalpy change (H) that occurs during a neutralization reaction between sodium hydroxide and one of three acids (HA) that will be assigned to you. HnA + nNaOH NanA + nH2O The calorimeter will be a “coffee cup” calorimeter, using two ...
Wanganui High School
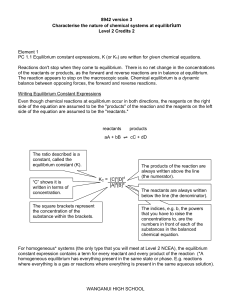
... 8942 version 3 Characterise the nature of chemical systems at equilibrium Level 2 Credits 2 ...
... 8942 version 3 Characterise the nature of chemical systems at equilibrium Level 2 Credits 2 ...
2. The Thermopile
... Thermodynamics provides a means for describing the observed thermoelectric properties; however it does not provide a model which can explain the mechanisms responsible for their behavior. The required model follows from an understanding of the roles of electrons in thermoelectric behavior. The relat ...
... Thermodynamics provides a means for describing the observed thermoelectric properties; however it does not provide a model which can explain the mechanisms responsible for their behavior. The required model follows from an understanding of the roles of electrons in thermoelectric behavior. The relat ...
10 PRE-LABORATORY ASSIGNMENT EXPERIMENT 7 1. Is t
... * R.C. Weast, ed., Handbook of Chemistry and Physics, 61st Ed., C.R.C. Press, Inc., 1980, pp. D67‐D78 (converted from kcal/mol) ...
... * R.C. Weast, ed., Handbook of Chemistry and Physics, 61st Ed., C.R.C. Press, Inc., 1980, pp. D67‐D78 (converted from kcal/mol) ...
Second Law of Thermodynamics
... specific heat of the gas—it had limited efficiency, defined as e = W /Qh , where W is the net work done by the engine and Qh is the quantity of heat put into the engine at a (high) temperature Th . Furthermore, he showed that the engine must necessarily put an amount of heat |Qc | back into a heat r ...
... specific heat of the gas—it had limited efficiency, defined as e = W /Qh , where W is the net work done by the engine and Qh is the quantity of heat put into the engine at a (high) temperature Th . Furthermore, he showed that the engine must necessarily put an amount of heat |Qc | back into a heat r ...
The Patent Officer - University of Leicester
... this energy, without the use of technically complex and fragile solar cells. The Johnson Converter consists of a chamber of air – just plain air, so no poisonous gases, no special materials that cost extra money, or can be exhausted and need to be replaced – with a piston to extract the energy. Esse ...
... this energy, without the use of technically complex and fragile solar cells. The Johnson Converter consists of a chamber of air – just plain air, so no poisonous gases, no special materials that cost extra money, or can be exhausted and need to be replaced – with a piston to extract the energy. Esse ...
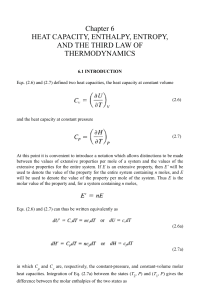
1 CHAPTER 8 HEAT CAPACITY, AND THE EXPANSION OF GASES
... If the heat is added at constant volume, we have simply that dU = dQ = CV dT. One other detail that requires some care is this. The specific heat capacity of a substance may well vary with temperature, even, in principle, over the temperature range of one degree mentioned in our definitions. Therefo ...
... If the heat is added at constant volume, we have simply that dU = dQ = CV dT. One other detail that requires some care is this. The specific heat capacity of a substance may well vary with temperature, even, in principle, over the temperature range of one degree mentioned in our definitions. Therefo ...
PDF (Chapter 5. Thermodynamics and Equations of State)
... probably can be treated with classical solid-state physics concepts. I say "probably" because the interior of the Earth is at simultaneous high temperature and high pressure and these are competing effects. The quantization of lattice vibrations and the departures from classical behavior that are of ...
... probably can be treated with classical solid-state physics concepts. I say "probably" because the interior of the Earth is at simultaneous high temperature and high pressure and these are competing effects. The quantization of lattice vibrations and the departures from classical behavior that are of ...
Chapter 19 - public.asu.edu
... Spontaneous - occurs without external intervention Non-Spontaneous - does not occur unless energy is added from an external source Equilibrium - not all reactions go to completion; reversible reactions (can move back and forth along the same path) ...
... Spontaneous - occurs without external intervention Non-Spontaneous - does not occur unless energy is added from an external source Equilibrium - not all reactions go to completion; reversible reactions (can move back and forth along the same path) ...
PDF only - at www.arxiv.org.
... negative over a wide range of α. This case points out the importance of coupled ionization and acceleration-induced concentration gradients on altering the average hydrodynamic profiles in an ICF plasma by greatly reducing and possibly reversing the direction of conductive heat flow for a mixed spe ...
... negative over a wide range of α. This case points out the importance of coupled ionization and acceleration-induced concentration gradients on altering the average hydrodynamic profiles in an ICF plasma by greatly reducing and possibly reversing the direction of conductive heat flow for a mixed spe ...
The International Association for the Properties of Water and Steam
... The upper limit of validity, the critical locus, can be found using the phase equilibrium criteria within the context of the present formulation. However, there may be convergence problems when an implementation of the formulation is used to calculate the critical line. Additional information about ...
... The upper limit of validity, the critical locus, can be found using the phase equilibrium criteria within the context of the present formulation. However, there may be convergence problems when an implementation of the formulation is used to calculate the critical line. Additional information about ...