Chemical Thermodynamics
... expansion work (P V work) that the system can do. Unless there is a change in the number of moles of gas present, this difference is extremely small and can usually be neglected. For an ideal gas, PV = nRT. At constant temperature and pressure, P V = (n)RT, a work term. Substituting gives: H = ...
... expansion work (P V work) that the system can do. Unless there is a change in the number of moles of gas present, this difference is extremely small and can usually be neglected. For an ideal gas, PV = nRT. At constant temperature and pressure, P V = (n)RT, a work term. Substituting gives: H = ...
The First Law of Thermodynamics Stephen Lower (2005) "Energy
... There is an important sign convention for heat and work that you are expected to know. If heat flows into a system or the surroundings to do work on it, the internal energy increases and the sign of q or w is positive. Conversely, heat flow out of the system or work done by the system will be at the ...
... There is an important sign convention for heat and work that you are expected to know. If heat flows into a system or the surroundings to do work on it, the internal energy increases and the sign of q or w is positive. Conversely, heat flow out of the system or work done by the system will be at the ...
LAW: The first law of thermodynamics states that the total energy in
... FL: Reversible and Irreversible Processes (take 2) ...
... FL: Reversible and Irreversible Processes (take 2) ...
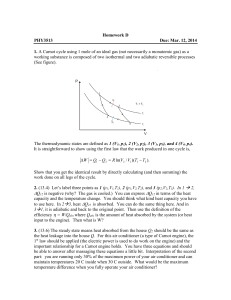
1 Basic problems for ideal gases and problems to the first law of
... from the surface (0th level) to 100 meters height? Mass of the stone is given by 500 kg. (Qdissipated =120.634 J, 3865 thermodynamic cycles) 2) A thermodynamic cycle is made with the working gas air. Pressure and temperature of the air is 1 bar and 27 C, respectively in the original status. The men ...
... from the surface (0th level) to 100 meters height? Mass of the stone is given by 500 kg. (Qdissipated =120.634 J, 3865 thermodynamic cycles) 2) A thermodynamic cycle is made with the working gas air. Pressure and temperature of the air is 1 bar and 27 C, respectively in the original status. The men ...
Basic Concepts of Thermodynamics
... c) The mean surface temperature T on Venus is a scorching 740K as compared to only 288K for Earth; the surface pressure is 90 times that on Earth. By what factor is the density of the near-surface Venusian atmosphere greater or less than that of Earth? 5. Two sealed containers with volumes V1 and V2 ...
... c) The mean surface temperature T on Venus is a scorching 740K as compared to only 288K for Earth; the surface pressure is 90 times that on Earth. By what factor is the density of the near-surface Venusian atmosphere greater or less than that of Earth? 5. Two sealed containers with volumes V1 and V2 ...
HormonesCascade
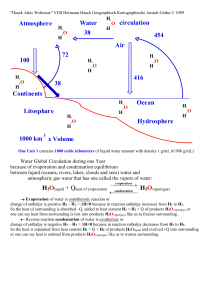
... So the heat of surrounding is absorbed –Q, added to heat content H2 = H1 + Q of products H2Ovapor(gas) or one can say heat from surrounding is lost into products H2Ovapor(gas) like as to freezes surrounding . Reverse reaction condensation of water is exothermic or change of enthalpy is negative H2 ...
... So the heat of surrounding is absorbed –Q, added to heat content H2 = H1 + Q of products H2Ovapor(gas) or one can say heat from surrounding is lost into products H2Ovapor(gas) like as to freezes surrounding . Reverse reaction condensation of water is exothermic or change of enthalpy is negative H2 ...