Solutions to TI4: First Law of Thermodynamics
... To discuss the flow of energy in or out of a system we first have to define the system we are talking about. A system may or may not be an isolated system. An isolated system is one where no energy flows in or out of the system. Consider the situation where Brent and Rebecca are sitting on the beach ...
... To discuss the flow of energy in or out of a system we first have to define the system we are talking about. A system may or may not be an isolated system. An isolated system is one where no energy flows in or out of the system. Consider the situation where Brent and Rebecca are sitting on the beach ...
Unit 12 - HKU Physics
... Imagine a container that is a cube of length L on a side. Its volume, then, is V = L3. In addition, consider a given molecule of mass m that moves in the negative x direction toward a wall. If its velocity is −vx and then vx after rebound from the container’s wall. The change of momentum of the mole ...
... Imagine a container that is a cube of length L on a side. Its volume, then, is V = L3. In addition, consider a given molecule of mass m that moves in the negative x direction toward a wall. If its velocity is −vx and then vx after rebound from the container’s wall. The change of momentum of the mole ...
Chapter 15
... first law of thermodynamics the heat lost by a system is equal to the heat gained by the system minus any work done by the system; conservation of energy heat engine device which changes internal energy into mechanical work isobaric any process in which the pressure of a gas remains constant isochor ...
... first law of thermodynamics the heat lost by a system is equal to the heat gained by the system minus any work done by the system; conservation of energy heat engine device which changes internal energy into mechanical work isobaric any process in which the pressure of a gas remains constant isochor ...
Chapter 15
... first law of thermodynamics the heat lost by a system is equal to the heat gained by the system minus any work done by the system; conservation of energy heat engine device which changes internal energy into mechanical work isobaric any process in which the pressure of a gas remains constant isochor ...
... first law of thermodynamics the heat lost by a system is equal to the heat gained by the system minus any work done by the system; conservation of energy heat engine device which changes internal energy into mechanical work isobaric any process in which the pressure of a gas remains constant isochor ...
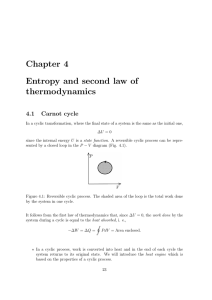
Chapter 4 Entropy and second law of thermodynamics
... As an illustration, let us consider a class in the school, where all kids sit always in the same place. This situation can be defined as ”ordered” since there is a strong correlation between the kids’ positions. For example, if Peter sits in the first row, you know straight away who is sitting behin ...
... As an illustration, let us consider a class in the school, where all kids sit always in the same place. This situation can be defined as ”ordered” since there is a strong correlation between the kids’ positions. For example, if Peter sits in the first row, you know straight away who is sitting behin ...