File - Elements of Mechanical Engineering
... Q9. A reversible engine working in a cycle takes 4500 kJ of heat from a source at 750K per minute and develops a power of 15kw. The engine rejects heat to two reservoirs at 300K and 400K. Determine the thermal efficiency and heat rejected to each sink. Q10. Two Carnot engines work in series between ...
... Q9. A reversible engine working in a cycle takes 4500 kJ of heat from a source at 750K per minute and develops a power of 15kw. The engine rejects heat to two reservoirs at 300K and 400K. Determine the thermal efficiency and heat rejected to each sink. Q10. Two Carnot engines work in series between ...
Overview
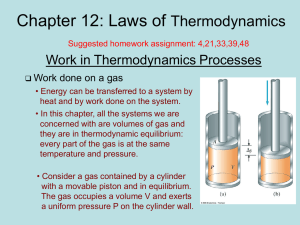
... to expand rapidly into a region of lower pressure. The rapid expansion does not allow time for heat to significantly enter the expanding gas. (A system that is so insulated from heat transfers is called an adiabatic system.) ...
... to expand rapidly into a region of lower pressure. The rapid expansion does not allow time for heat to significantly enter the expanding gas. (A system that is so insulated from heat transfers is called an adiabatic system.) ...
Chapter 4 - UniMAP Portal
... is very inconvenient for hand calculations but quite desirable for computerized calculations. The results obtained are very accurate. 3. By using average specific heats. This is very simple and certainly very Three ways of calculating u. convenient when property tables are not available. The result ...
... is very inconvenient for hand calculations but quite desirable for computerized calculations. The results obtained are very accurate. 3. By using average specific heats. This is very simple and certainly very Three ways of calculating u. convenient when property tables are not available. The result ...
lecture1424085736
... Examples: 1. Frictionless isothermal expansion or compression of a fluid. 2. Frictionless adiabatic expansion or compression of a fluid. 3. Elastic stretching of a solid. 4. Electric current with zero resistance. IRREVERSIBLE PROCESS An irreversible process is one that is carried out in such a way t ...
... Examples: 1. Frictionless isothermal expansion or compression of a fluid. 2. Frictionless adiabatic expansion or compression of a fluid. 3. Elastic stretching of a solid. 4. Electric current with zero resistance. IRREVERSIBLE PROCESS An irreversible process is one that is carried out in such a way t ...
Lecture12
... In a car engine operating at 1.80x103 rev/min, the expansion of hot, high-pressure gas against a piston occurs in about 10 ms. because energy transfer by heat typically takes a time on the order of minutes or hours, it is safe to assume that little energy leaves the hot gas during expansion. Estimat ...
... In a car engine operating at 1.80x103 rev/min, the expansion of hot, high-pressure gas against a piston occurs in about 10 ms. because energy transfer by heat typically takes a time on the order of minutes or hours, it is safe to assume that little energy leaves the hot gas during expansion. Estimat ...
Problem 1 Separation of gases
... b) Use your model to calculate the minimum work input for separation of 1 kmol pure CO2 from from a flue gas containing 1) 3,5% CO2 (typically gas turbine) 2) 11% CO2 (typically gas engine or coalfired power plant), when in both cases the degree of separation ( β CO2 ) ranges from 0,3 to 0,9. The en ...
... b) Use your model to calculate the minimum work input for separation of 1 kmol pure CO2 from from a flue gas containing 1) 3,5% CO2 (typically gas turbine) 2) 11% CO2 (typically gas engine or coalfired power plant), when in both cases the degree of separation ( β CO2 ) ranges from 0,3 to 0,9. The en ...
Lecture 2 - Richard Grotjahn
... • T =Temperature, P =Pressure, R=constant • Density ρ = mass / volume = M / Vol • Equation: P=ρRT=MRT Vol • T of air inside decreases • Mass M of air inside does not change. • P outside, pushing on jug does not change • So, V decreases to match decrease of T ...
... • T =Temperature, P =Pressure, R=constant • Density ρ = mass / volume = M / Vol • Equation: P=ρRT=MRT Vol • T of air inside decreases • Mass M of air inside does not change. • P outside, pushing on jug does not change • So, V decreases to match decrease of T ...