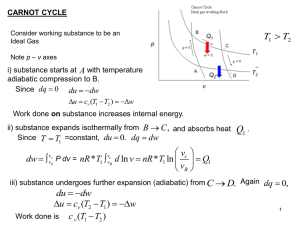
Name: SOLUTIONS Physics 240, Exam #1 Sept. 24 2015 (4:15
... 5 POINTS TOTAL (5 points) SOLUTION We need two Key Ideas here. One is that we can determine the entropy change for the irreversible process by calculating it for a reversible process that provides the same change in volume. The other is that the temperature of the gas does not change in the free exp ...
... 5 POINTS TOTAL (5 points) SOLUTION We need two Key Ideas here. One is that we can determine the entropy change for the irreversible process by calculating it for a reversible process that provides the same change in volume. The other is that the temperature of the gas does not change in the free exp ...
Thermodynamics - TCD Maths home
... Definition: (Equilibrium State, State Variables) An equilibrium state is one in which all the bulk physical properties of the system are uniform throughout the system and do not change with time. An equilibrium state is specified by two independent variables known as state variables. Definition: (Th ...
... Definition: (Equilibrium State, State Variables) An equilibrium state is one in which all the bulk physical properties of the system are uniform throughout the system and do not change with time. An equilibrium state is specified by two independent variables known as state variables. Definition: (Th ...
Document
... external pressure is suddenly decreased by ΔP, a nonequilibrium state is induced, so the piston will move outward causing a pressure drop inside the cylinder, thus the liquid water evaporate in a process of establishment mechanical equilibrium by equalization of the external and internal pressure. T ...
... external pressure is suddenly decreased by ΔP, a nonequilibrium state is induced, so the piston will move outward causing a pressure drop inside the cylinder, thus the liquid water evaporate in a process of establishment mechanical equilibrium by equalization of the external and internal pressure. T ...
PHYSICAL SCIENCE CHAPTER 3 STATES OF MATTER
... Objects with a density ___________________ than water will ________________________. 6. Although steel has a density almost _____ times that of water, a steel ship will float if its shape causes it to displace a volume of water greater than its weight thus changing the ship’s density. B. Fluids and ...
... Objects with a density ___________________ than water will ________________________. 6. Although steel has a density almost _____ times that of water, a steel ship will float if its shape causes it to displace a volume of water greater than its weight thus changing the ship’s density. B. Fluids and ...
Midterm Exam Problem 10 Example of using van der Waals
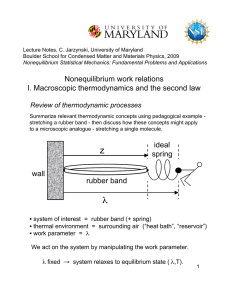
... uniquely on the state of a system makes it useful. Like internal energy U, S is a property that is not obvious, but needs to be calculated from other properties of the system. If volume V were such a nonobvious property, how could we discover it? Consider as the system an ideal gas in a cylinder con ...
... uniquely on the state of a system makes it useful. Like internal energy U, S is a property that is not obvious, but needs to be calculated from other properties of the system. If volume V were such a nonobvious property, how could we discover it? Consider as the system an ideal gas in a cylinder con ...
Pressure and Density and the Temperature
... changes in pressure and volume, via the gas law. pV=nRT ...
... changes in pressure and volume, via the gas law. pV=nRT ...
PX121: Thermal Physics Lecture 2
... “A system in equilibrium reacts to a (small) externally imposed change in one of its state variables by readjusting its internal condition so as to reverse the change.” (Henri Louis le Chatelier, 1844) E.g. ...
... “A system in equilibrium reacts to a (small) externally imposed change in one of its state variables by readjusting its internal condition so as to reverse the change.” (Henri Louis le Chatelier, 1844) E.g. ...