* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Work done by the system
Calorimetry wikipedia , lookup
Van der Waals equation wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Internal energy wikipedia , lookup
Equation of state wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Thermodynamic system wikipedia , lookup
History of thermodynamics wikipedia , lookup
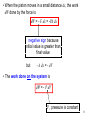
The study of relationships involving heat, mechanical work, and other aspects of energy and energy transfer for the system. CHAPTER 15: Thermodynamics (4 Hours) Thermodynamic system is any collection of objects that is convenient to regard as a unit, and that may have the potential energy to exchange energy with its surroundings. SUBTOPIC 15.1 First Law of Thermodynamics 15.2 Thermodynamics Processes 15.3 Thermodynamics Work 2 Learning Outcome: 15.1 First law of thermodynamics (1 hour) At the end of this chapter, students should be able to: Distinguish between work done on the system and work done by the system. State and use first law of thermodynamics, Q U W 3 15.1 First Law of Thermodynamics • Thermodynamics is the study of energy relationships that involve heat, mechanical work, and other aspects of energy and energy transfer. • The first law of thermodynamics is the extension of the principle of conservation of energy to include both heat and mechanical energy. • 3 quantities involved in a thermodynamic system : 1. Heat , Q 2. Internal energy , ΔU 3. Work , W 4 Work done by the system (+) Initial A Gas dx Final F A • When a gas expands, its pushes out on its boundary surfaces as they move outward; an expanding gas always does positive work. 5 • Figure above shows a gas in a cylinder with a moveable piston. • Suppose that the cylinder has a cross sectional area, A and the pressure exerted by the gas (system) at the piston face is P. • The force F exerted on the piston by the system is F=PA. • When the piston moves out a small distance dx, the work dW done by the force is dW = F dx = PA dx but A dx = dV dV is the small change of volume of the system (gas) • The work done by the system is dW = P dV P , pressure is constant 6 Work done on the system (-) • Suppose that the cylinder has a cross sectional area, A and the pressure of the gas is P. F • The external force F exerted on the system is F=PA • The magnitude of external force F exerted on the system equal to PA because the piston is always in equilibrium between the external force and the force from the gas. dx 7 • When the piston moves in a small distance dx, the work dW done by the force is dW = - F dx = -PA dx negative sign because initial value is greater than final value but - A dx = - dV • The work done on the system is dW = - P dV P , pressure is constant 8 • In both cases if the volume of the gas changes from V1 to V2 , the work done is given by dW P dV V2 dW V1 PdV W PV2 V1 W PV Work done by the system • Gas expands • Volume increases •+W W : work done P : gas pressure V1 : initial volume V2 : final volume W = - PV Work done on the system • Gas is compressed • Volume decreases •-W 9 The First Law of Thermodynamics (flot) “ The change in internal energy of a closed system, ΔU, will be equal to the heat added to the system minus the work done by the system “ U Q W + U : increase in internal energy - U : decrease in internal energy +Q + Q : heat is added to the system - Q : heat is removed from the system -W + W : work is done by the system - W : work is done on the system -Q system +W 10 U Q W Rearrange Q = U + W Translation : • When Q is added to a system (gas) , the temperature of the gas increases, thus causing the internal energy to increase by an amount of ΔU joule. • At the same time, when its temperature increases, its volume increases too. • When the volume of the gas increases, work is done by the gas (W). 11 12 Example 15.1 A 2500 J heat is added to a system and 1800 J work is done on the system. Calculate the change in internal energy of the system. Solution 13 Example 15.2 The work done to compress one mole of a monoatomic ideal gas is 6200 J. The temperature of the gas changes from 350 to 550 K. a) How much heat flows between the gas and its surroundings ? b) Determine whether the heat flows into or out of the gas. Solution 14 Solution 15.2 n 1, f 3, W 6200 J T1 350 K , T2 550 K a) 15 Example 15.3 A gas in a cylinder expands from a volume of 0.400 m3 to 0.700 m3. Heat is added just rapidly enough to keep the pressure constant at 2.00 x 105 Pa during the expansion. The total heat added is 1.40 x 105 J. Calculate the work done by the gas and the change in internal energy of the gas. Solution 16 Exercise 1. A system absorbs 200 J of heat as the internal energy increases by 150 J. What work is done by the gas ? 50 J 2. In a chemical laboratory, a technician applies 340 J of energy to a gas while the system surrounding the gas does 140 J of work on the gas. What is the change in internal energy ? 480 J 3. 8000 J of heat is removed from a refrigerator by a compressor which has done 5000 J of work. What is the change in internal energy of the gas in the system? -3000 J 17 Learning Outcome: 15.2 Thermodynamics processes (1 hour) At the end of this chapter, students should be able to: Define the following thermodynamics processes: Isothermal, ΔU= 0 Isovolumetric, W = 0 Isobaric, ΔP = 0 Adiabatic, Q = 0 Sketch PV graph for all the thermodynamic processes. 18 15.2 Thermodynamics processes • There are 4 common processes of thermodynamics: 1) 2) 3) 4) Isothermal process Isochoric (isovolumetric) process Isobaric process Adiabatic process (“iso” = same) T V,P 19 1) Isothermal process • Isothermal process is defined as a process that occurs at constant temperature. • U 0 U = U 2 - U1 T1 = T2 f f U = nRT2 - nRT1 = 0 2 2 From flot, U Q W If U = 0 then Q =W • From the Boyle’s law : PV = constant P1V1 P2V2 20 2) Isochoric (isovolumetric) process • Isochoric (isovolumetric) process is defined as a process that occurs at constant volume. • W =0 W = P(V2 - V1 ) = 0 V1 = V2 From flot, U Q W If W = 0 then U = Q 21 3) Isobaric process • Isobaric process is defined as a process that occurs at constant pressure. From flot, U Q W U Q PV2 V1 W PV 4) Adiabatic process • Adiabatic process is defined as a process that occurs without the transfer of heat (into or out of the system). • Q=0 From flot, U Q W If Q = 0 then U = - W 22 Pressure-Volume Diagram (graph) for Thermodynamic Processes P T4 > T3 > T2 > T1 A PA D 0 Path AB Path AC Path AD Path AE VA E B C T4 T3 T2 T1 V Isothermal process (TB=TA) Adiabatic process (TC<TA) Isochoric process (TD<TA) Isobaric process (TE>TA) 23 Exercise 1. A gas system which undergoes an adiabatic process does 5.0kJ of work against an external force. What is the change in its internal energy? 5000 J 2. A gas is compressed under constant pressure, i) Sketch the pressure –volume graph. ii) How is the work done in compressing the gas calculated? iii) Explain what will happen to the final temperature of the gas. 3. A gas undergoes the following thermodynamics processes: isobaric expansion, heated at constant volume, compressed isothermally, and finally expands adiabatically back its initial pressure and volume. Sketch all the processes given on the same P-V graph. 24 Learning Outcome: 15.3 Thermodynamics work (2 hours) At the end of this chapter, students should be able to: Derive expression for work , W PdV Determine work from the area under the p-V graph Derive the equation of work done in isothermal, isovolumetric, and isobaric processes. Calculate work done in isothermal process and use V2 p nRT ln 1 V1 p2 isobaric process, use W PdV p(V2 V1 ) W nRT ln isovolumetric process, use W PdV 0 25 15.3 Thermodynamics work Work done in the thermodynamics system Consider the infinitesimal work done by the gas (system) during the small expansion, dx in a cylinder with a movable piston as shown in Figure 15.3. Initial A Gas dx Final F A Figure 15.3 Suppose that the cylinder has a cross sectional area, A and the pressure exerted by the gas (system) at the piston face is P. 26 The work, dW done by the gas is given by In a finite change of volume from V1 to V2, dW Fdx cos where 0 and F PA dW PAdx and Adx dV dW PdV V2 dW V1 V2 PdV W PdV (16.1) V1 where W : work done P : gas pressure V1 : initial volume of the gas V2 : final volume of the gas 27 PV diagram Work done = area under the P-V graph P P isothermal expansion P1 1 P2 0 W 0 V1 P2 2 V2 isobaric expansion 1 2 W P1 V2 V1 0 0 0 1 W 0 V2 V1 V P P P1 2 P1 V isothermal compression V1 V2 V P2 2 isochoric W 0 P1 1 0 V1 V 28 Equation of work done in thermodynamic processes 1) Isothermal U 0 U = Q - W = 0 Q =W nRT Q =W = ∫ PdV = ∫ dV V1 V1 V V2 From Boyle’s law : P1V1 P2V2 W = nRT ln V1 V2 P1 P1 W = nRT ln V1 P2 P2 V2 V2 29 2) Isochoric (isovolumetric) Since the volume of the system in isovolumetric process remains unchanged, thus dV 0 Therefore the work done in the isovolumetric process is W PdV 0 Work done at constant volume 3) Isobaric The work done during the isobaric process which change of volume from V1 to V2 is given by V2 W PdV V1 V2 and P constant W P dV W = PV = P(V2 - V1 ) V1 Work done at constant pressure 30 Example 15.4 How much work is done by an ideal gas in expanding isothermally from an initial volume of 3.00 liters at 20.0 atm to a final volume of 24.0 liters? Solution V1 = 3.00 liters, V2 = 24.0 liters , P = 20.0 atm 31 Example 15.5 Two liters of an ideal gas have a temperature of 300 K and a pressure of 20.0 atm. The gas undergoes an isobaric expansion while its temperature is increased to 500 K. What work is done by the gas ? Solution T1 = 300 K, T2 = 500 K , P = 20.0 atm, V1 =2 liters 32 Example 15.6 (a) Write an expression representing i. the 1st law of thermodynamics and state the meaning of all the symbols. ii. the work done by an ideal gas at variable pressure. [3 marks] (b) Sketch a graph of pressure P versus volume V of 1 mole of ideal gas. Label and show clearly the four thermodynamics process. [5 marks] (Exam.Ques.intake 2003/2004) 33 Solution 15.6 a) i. 1st law of thermodynamics: ii. Work done at variable pressure: or where 34 b) PV diagram below represents four thermodynamic processes: 35 Example 15.7 In a thermodynamic system, the changing of state for that system shows by the PV-diagram below. Px104 / Pa 8.0 B 3.0 0 A 2.0 D C 5.0 3 Vx10 / m 3 In process AB, 150 J of heat is added to the system and in process BD, 600 J of heat is added. Determine a. the change in internal energy in process AB. b. the change in internal energy in process ABD. c. the total heat added in process ACD. 36 Solution 15.7 QAB= 150 J, QBD= 600 J, VA=VB= 2.0x10-3 m3, VC=VD= 5.0x10-3 m3, PA=PC= 3x104 Pa, PB=PD= 8x104 Pa 37 4 Solution 15.7 Px10 / Pa QAB= 150 J, QBD= 600 J, 8.0 B VA=VB= 2.0x10-3 m3, VC=VD= 5.0x10-3 m3, PA=PC= 3x104 Pa, PB=PD= 8x104 Pa 3.0 0 b. The work done in process ABD is A 2.0 D C 5.0 Vx103 / m3 The total heat transferred in process ABD is given by Therefore, the change in internal energy : 38 4 Solution 15.7 Px10 / Pa QAB= 150 J, QBD= 600 J, 8.0 B VA=VB= 2.0x10-3 m3, VC=VD= 5.0x10-3 m3, PA=PC= 3x104 Pa, PB=PD= 8x104 Pa 3.0 0 c. The change in internal energy in process ACD is The work done in process ACD is given by A 2.0 D C 5.0 Vx103 / m Therefore, the total heat transferred : 39 Example 15.8 A gas in the cylinder of a diesel engine can undergo cyclic processes. Figure below shows one cycle ABCDA that is executed by an ideal gas in the engine mentioned. a. If the temperature of the gas in states A and B are 300 K and 660 K, respectively. Calculate the temperature in states C and D. b. Determine the work done by the gas in process BC. P / x10 5 Pa B 16.0 C D 7.8 A 1.0 0 1.40 6.00 10.00 V / x10 4 m 3 40 Solution 15.8 PB=PC= 16.0x105 Pa, PA= 1.0x105 Pa, PD= 7.8x105 Pa, VA=VD= 10.0x10-4 m3, VB= 1.40x10-4 m3, VC= 6.00x10-4 m3 a. Given TA= 300 K and TB= 660 K P / x10 5 Pa B 16.0 C D 7.8 A 1.0 0 1.40 6.00 10.00 V / x10414 m 3 Solution 15.8 PB=PC= 16.0x105 Pa, PA= 1.0x105 Pa, PD= 7.8x105 Pa, VA=VD= 10.0x10-4 m3, VB= 1.40x10-4 m3, VC= 6.00x10-4 m3 5 P / x10 Pa B 16.0 C D 7.8 A 1.0 0 1.40 6.00 10.00 V / x10 4 m 3 b. Process BC occurs at constant pressure, thus the work done by the gas is given by 42 Example 15.9 (a) Define (i) the adiabatic compression process (ii) the reversible process [2 marks] (b) One mole of an ideal monatomic gas is at the initial temperature of 650 K. The initial pressure and volume of the gas is P0 and V0, respectively. At initial stage, the gas undergoes isothermal expansion and its volume increase to 2V0. Then, this gas through the isochoric process and return to its initial pressure. Finally, the gas undergoes isobaric compression so that it return to its initial temperature, pressure and volume. (i) Sketch the pressure against volume graph for the entire process. [4 marks] (ii) By using the 1st law of thermodynamics, proved that the heat, Q nRT0 ln 2 P0V0 where n is the number of moles, R is molar gas constant and T is the absolute temperature. Then, calculate the total heat for the entire process. [8 marks] (iii) State whether the heat is absorbed or released. [1 mark] (Use R = 8.31 J K-1 mol-1) 43 (Exam.Ques.intake 2001/2002) Solution 15.9 44 Solution 15.9 (b) One mole of an ideal monatomic gas is at the initial temperature of 650 K. The initial pressure and volume of the gas is P0 and V0, respectively. At initial stage, the gas undergoes isothermal expansion and its volume increase to 2V0. Then, this gas through the isochoric process and return to its initial pressure. Finally, the gas undergoes isobaric compression so that it return to its initial temperature, pressure and volume. 45 Solution 15.9 (i) Sketch the pressure against volume graph for the entire process. Pressure, P P0 A C T1 B P1 0 T0 V0 2V0 Volume, V 46 Solution 15.9 ii. From the PV diagram, In process AB (Isothermal process): Pressure, P P0 A C T1 B P1 0 T0 V0 2V0 Volume, V In process CA (isobaric process): Using Charles’s law, hence 47 Solution15.9 The work done in isobaric process: Pressure, P P0 A C T1 B P1 0 T0 V0 2V0 Volume, V In process BC (isochoric process): 48 Solution 15.9 Pressure, P A P0 The total heat, Q for entire process is given by C T1 B P1 0 T0 V0 2V0 Volume, V ….. proved 49 Example 15.10 (a) State i. the isobaric process. ii. the isothermal process. iii. the adiabatic process. (b) State the 1st law of thermodynamics. [3 marks] [2 marks] 50 Solution 15.10 b. 1st law of thermodynamics : 51 Exercise Two moles of ideal gas are at a temperature of 300K and pressure 2.5 x 105 Pa. The gas expands isothermally to twice its initial volume, and then undergoes isobaric compression to its initial volume. i) ii) Calculate the initial volume of the gas. What is the pressure of the gas after the gas expands isothermally to twice its initial volume? iii) What is the final temperature of the gas after being compressed isobarically? iv) Calculate the work done in the isothermal expansion. v) Draw the P-V graph for the processes above. 0.02 m3,1.3 x 105 Pa, 150 K, 3.5 x 103 J 52 Good luck For 1st semester examination 53