Chapter 2
... Define and distinguish among atomic number, mass number, atomic weight, and valence. Given the atomic number and mass number of an atom, how do you determine the number of its neutrons? ___6. Explain why radioactive isotopes are important to biologists. ___7. Explain how its electron configuration i ...
... Define and distinguish among atomic number, mass number, atomic weight, and valence. Given the atomic number and mass number of an atom, how do you determine the number of its neutrons? ___6. Explain why radioactive isotopes are important to biologists. ___7. Explain how its electron configuration i ...
Name
... Essential Standard 5b: The idea of atoms explains the conservation of matter: in chemical reactions the number of atoms stays the same no matter how they are arranged, so their total mass stays the same. ...
... Essential Standard 5b: The idea of atoms explains the conservation of matter: in chemical reactions the number of atoms stays the same no matter how they are arranged, so their total mass stays the same. ...
Electronic Structure Calculations of InP
... model has been used [10, 11]. It has several functionalities necessary for a proper calculation of this rather complicated quantum structure. The program is able to calculate the energy levels of a QW in a 3D geometric numerical box and it can simultaneously handle QDs of even large sizes, with diff ...
... model has been used [10, 11]. It has several functionalities necessary for a proper calculation of this rather complicated quantum structure. The program is able to calculate the energy levels of a QW in a 3D geometric numerical box and it can simultaneously handle QDs of even large sizes, with diff ...
name chemistry final review
... How is ionic radius different from atomic radius? Be specific. Cations are smaller than their parent atoms because they have lost electrons and anions are larger than their parent atoms since they have gained electrons. ...
... How is ionic radius different from atomic radius? Be specific. Cations are smaller than their parent atoms because they have lost electrons and anions are larger than their parent atoms since they have gained electrons. ...
Reaction types summary
... The species which causes oxidation is called the oxidizing agent. The substance which is oxidized loses electrons to the other. The oxidizing agent is always reduced ...
... The species which causes oxidation is called the oxidizing agent. The substance which is oxidized loses electrons to the other. The oxidizing agent is always reduced ...
PowerPoint material for lecture 1 (September 4, 2012)
... • In bulk solids, all molecules are surrounded by and bound to neighboring atoms and the forces are in balance. Surface atoms are bound only on one side, leaving unbalanced atomic and molecular forces on the surface. These forces attract gases and molecules Van der Waals force, physical adsorpti ...
... • In bulk solids, all molecules are surrounded by and bound to neighboring atoms and the forces are in balance. Surface atoms are bound only on one side, leaving unbalanced atomic and molecular forces on the surface. These forces attract gases and molecules Van der Waals force, physical adsorpti ...
4.4 Oxidation Reduction Redox An introduction to
... The species which causes oxidation is called the oxidizing agent. The substance which is oxidized loses electrons to the other. The oxidizing agent is always reduced ...
... The species which causes oxidation is called the oxidizing agent. The substance which is oxidized loses electrons to the other. The oxidizing agent is always reduced ...
C. - Biloxi Public Schools
... • Heisenberg showed it is impossible to take any measurement of an object without disturbing it. • The Heisenberg uncertainty principle states that it is fundamentally impossible to know precisely both the velocity and position of a particle at the same time. • The only quantity that can be known is ...
... • Heisenberg showed it is impossible to take any measurement of an object without disturbing it. • The Heisenberg uncertainty principle states that it is fundamentally impossible to know precisely both the velocity and position of a particle at the same time. • The only quantity that can be known is ...
Measurement of Surface Quality 1. Lyot Test 2. FECO 3. Nomarski
... of the variation of d in that section since there is exact point-to-point correspondence between the selected region and its image on the slit. Small changes in d are determined by measuring small changes in O. There are no ambiguities as to whether a region is a hill or a valley. There are no ambig ...
... of the variation of d in that section since there is exact point-to-point correspondence between the selected region and its image on the slit. Small changes in d are determined by measuring small changes in O. There are no ambiguities as to whether a region is a hill or a valley. There are no ambig ...
FIB – an easy tool for fabrication of high quality plasmonic structures
... photonic data storage, light generation, and bio-photonics. SPPs cannot be excited directly by incident electromagnetic radiation. One of the possible ways for their excitation is scattering of an incident electromagnetic wave on surface perturbations: holes, grooves and slits. In this example, an o ...
... photonic data storage, light generation, and bio-photonics. SPPs cannot be excited directly by incident electromagnetic radiation. One of the possible ways for their excitation is scattering of an incident electromagnetic wave on surface perturbations: holes, grooves and slits. In this example, an o ...
Elements, their Symbol, Atomic Number and Molar Mass
... 2. I had announced that atoms of a given element are identical in mass and chemical properties. 3. I am the building block of an element. 4. I represent sodium as Na. 5. I am the smallest unit that gives the relative mass of an atom. 6. I am a monoatomic molecule. 7. I am a giant molecule containing ...
... 2. I had announced that atoms of a given element are identical in mass and chemical properties. 3. I am the building block of an element. 4. I represent sodium as Na. 5. I am the smallest unit that gives the relative mass of an atom. 6. I am a monoatomic molecule. 7. I am a giant molecule containing ...
SOME ELEMENTS OF ATOMIC STRUCTURE THEORY
... and are thus a very convenient label for atomic energy levels. For example, in an excited state of helium, we may have the configuration 1s2p, so that there are four angular momenta to combine: l1 = 0, l2 = 1, s1 = s2 = 1/2. We know there will be 12 possible states, since there are three possible val ...
... and are thus a very convenient label for atomic energy levels. For example, in an excited state of helium, we may have the configuration 1s2p, so that there are four angular momenta to combine: l1 = 0, l2 = 1, s1 = s2 = 1/2. We know there will be 12 possible states, since there are three possible val ...
Term 1 and 2 Powerpoints
... submitted per unit. You choose the lab you want to do the report on. It is suggested that you choose one at the beginning of the unit so that you can get it done ahead of time. It will be due day of test. • Leave 5 minutes at the end of each period for clean up. Labs are not expected to be homework. ...
... submitted per unit. You choose the lab you want to do the report on. It is suggested that you choose one at the beginning of the unit so that you can get it done ahead of time. It will be due day of test. • Leave 5 minutes at the end of each period for clean up. Labs are not expected to be homework. ...
Theory of longitudinal magnetoresistance in weak magnetic fields
... crystal, s o that no resistance change due to crystalline anisotropy takes place. The magnetoresistance i s z e r o because the action of the magnetic field on the electron system is taken into account only classically-via the Lorentz force. According to quantum mechanics, a system of Landau levels ...
... crystal, s o that no resistance change due to crystalline anisotropy takes place. The magnetoresistance i s z e r o because the action of the magnetic field on the electron system is taken into account only classically-via the Lorentz force. According to quantum mechanics, a system of Landau levels ...
Regents Chemistry Topic Review Packet
... You can recognize an excited state electron configuration. If the configuration does not match that on the Periodic Table for that number of electrons, then it is an excited state. 9. When an electron returns from a higher energy state to a lower energy state, it emits a specific amount of energy ...
... You can recognize an excited state electron configuration. If the configuration does not match that on the Periodic Table for that number of electrons, then it is an excited state. 9. When an electron returns from a higher energy state to a lower energy state, it emits a specific amount of energy ...
Name - Madison County Schools
... 15) What could happen if you point the end of a test tube that you’re heating towards someone? Material could shoot out the end and hit someone. 16) What’s the best way to pick up a piece of glassware you’ve been heating? With tongs ...
... 15) What could happen if you point the end of a test tube that you’re heating towards someone? Material could shoot out the end and hit someone. 16) What’s the best way to pick up a piece of glassware you’ve been heating? With tongs ...
Chemical formulae Worksheet
... CHEMICAL FORMULAE AND EQUATIONS ionic bonds = electrons have been transferred between atoms, resulting in oppositely charged ions that attract each other. Contain cations and anions. covalent bonds = two atoms share some of their electrons. Atomic element = one atom e.g. Ne, Xe Molecular element = e ...
... CHEMICAL FORMULAE AND EQUATIONS ionic bonds = electrons have been transferred between atoms, resulting in oppositely charged ions that attract each other. Contain cations and anions. covalent bonds = two atoms share some of their electrons. Atomic element = one atom e.g. Ne, Xe Molecular element = e ...
Fundamentals of General Chemistry and Physical Chemistry for
... Kohlrausch's law of independent migration of ions: Each ion is assumed to make its own contribution to the molar conductivity, irrespective of the nature of the other ion with which it is associated. ...
... Kohlrausch's law of independent migration of ions: Each ion is assumed to make its own contribution to the molar conductivity, irrespective of the nature of the other ion with which it is associated. ...
Nugget
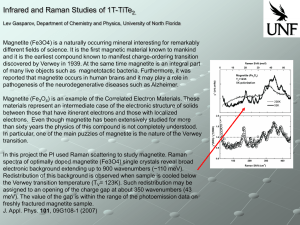
... discovered by Verwey in 1939. At the same time magnetite is an integral part of many live objects such as magnetotactic bacteria. Furthermore, it was reported that magnetite occurs in human brains and it may play a role in pathogenesis of the neurodegenerative diseases such as Alzheimer. Magnetite ( ...
... discovered by Verwey in 1939. At the same time magnetite is an integral part of many live objects such as magnetotactic bacteria. Furthermore, it was reported that magnetite occurs in human brains and it may play a role in pathogenesis of the neurodegenerative diseases such as Alzheimer. Magnetite ( ...
Chemistry I Exams and Answer Keys 2015 Season
... [Note: 1 nm (nanometer) = 10–9 m] A. lower energy. B. a greater velocity. C. a higher frequency. D. a longer wavelength. ...
... [Note: 1 nm (nanometer) = 10–9 m] A. lower energy. B. a greater velocity. C. a higher frequency. D. a longer wavelength. ...
Design and Characterization of a Novel Hybrid-field Ion
... A novel design of a Radio-Frequency (RF) octapole ion guide arrangement forming multiple longitudinal segments is described using gas dynamics calculations and ion optics simulations. The ion guide is constructed and further evaluated experimentally on a prototype orthogonal Time-of-Flight Mass Spec ...
... A novel design of a Radio-Frequency (RF) octapole ion guide arrangement forming multiple longitudinal segments is described using gas dynamics calculations and ion optics simulations. The ion guide is constructed and further evaluated experimentally on a prototype orthogonal Time-of-Flight Mass Spec ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.