
9. Balancing Equations
... Which says: Matter cannot be created or destroyed; therefore, You must have the same number of atoms on each side of a chemical equation to show conservation of mass. ie. Reactants = Products ...
... Which says: Matter cannot be created or destroyed; therefore, You must have the same number of atoms on each side of a chemical equation to show conservation of mass. ie. Reactants = Products ...
Micron-scale modifications of Si surface morphology by pulsed
... experiment and theory because we have little knowledge of how changes in the microstructure and desorption of the oxide layer alters the surface tension of the laser melt.12 For these reasons, we compare laser texturing before and after treatment with hydrofluoric 共HF兲 acid. First, dimples are produ ...
... experiment and theory because we have little knowledge of how changes in the microstructure and desorption of the oxide layer alters the surface tension of the laser melt.12 For these reasons, we compare laser texturing before and after treatment with hydrofluoric 共HF兲 acid. First, dimples are produ ...
File
... Relative Atomic Mass of elements The atoms of each element have a different mass. Carbon is given a relative atomic mass (RAM) of 12. The RAM of other atoms compares them with carbon. Eg. Hydrogen has a mass of only one twelfth that of carbon and so has a RAM of 1. Below are the RAMs of some other ...
... Relative Atomic Mass of elements The atoms of each element have a different mass. Carbon is given a relative atomic mass (RAM) of 12. The RAM of other atoms compares them with carbon. Eg. Hydrogen has a mass of only one twelfth that of carbon and so has a RAM of 1. Below are the RAMs of some other ...
Chemical Composition
... 4. Use this as a conversion factor for moles-to-grams Molar Mass The molar mass is the mass in grams of one mole of a compound The relative weights of molecules can be calculated from atomic masses water = H2O = 2(1.008 amu) + 16.00 amu = 18.02 amu 1 mole of H2O will weigh 18.02 g, therefore ...
... 4. Use this as a conversion factor for moles-to-grams Molar Mass The molar mass is the mass in grams of one mole of a compound The relative weights of molecules can be calculated from atomic masses water = H2O = 2(1.008 amu) + 16.00 amu = 18.02 amu 1 mole of H2O will weigh 18.02 g, therefore ...
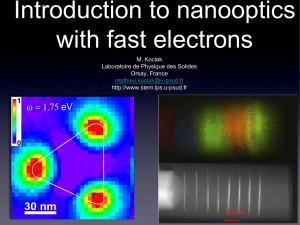
EELS
... ‣One can show that the EELS is a very good approximation of the EMLD ‣EMLDOS is a very concise description of optical excitations ‣The EELS is a map of the plasmons “eigenfunction” (with some marks... ‣The STEM-EELS is a decent plasmonic counterpart of the STM for ...
... ‣One can show that the EELS is a very good approximation of the EMLD ‣EMLDOS is a very concise description of optical excitations ‣The EELS is a map of the plasmons “eigenfunction” (with some marks... ‣The STEM-EELS is a decent plasmonic counterpart of the STM for ...
Basic Constituents of Matter and their Interactions
... that the smaller the distance you want to probe the higher must be the beam energy. Thus probing inside the proton (x ≪ 1f m) requires a beam energy E ≫ 1 GeV(109 eV), which is the energy acquired by the electron on passing through a billion (Gega) volts. It is this multi-GeV acceleration technique ...
... that the smaller the distance you want to probe the higher must be the beam energy. Thus probing inside the proton (x ≪ 1f m) requires a beam energy E ≫ 1 GeV(109 eV), which is the energy acquired by the electron on passing through a billion (Gega) volts. It is this multi-GeV acceleration technique ...
OSA journals template (MSWORD) - HAL
... 100 Hz with 45 fs duration. The beam 1/e2 radius is 6.8 mm and polarization is along y (TE). We constructed a planar waveguide using two fused silica slabs (21 cm long, 1.9 cm wide and 0.6 cm thick) separated by 127 µm thick plastic spacers. The waveguide is kept in a 2 m long gas cell with anti-ref ...
... 100 Hz with 45 fs duration. The beam 1/e2 radius is 6.8 mm and polarization is along y (TE). We constructed a planar waveguide using two fused silica slabs (21 cm long, 1.9 cm wide and 0.6 cm thick) separated by 127 µm thick plastic spacers. The waveguide is kept in a 2 m long gas cell with anti-ref ...
Grade 10 NSC Chemistry Curriculum
... Students should develop an understanding about the importance of the periodic table in Chemistry. Knowledge and concepts about periodic trends of physical properties of some elements are required • Understand that elements in the PT are arranged in order of ascending atomic number • Appreciate the P ...
... Students should develop an understanding about the importance of the periodic table in Chemistry. Knowledge and concepts about periodic trends of physical properties of some elements are required • Understand that elements in the PT are arranged in order of ascending atomic number • Appreciate the P ...
Unit 3: Bonding and Nomenclature Content Outline: Chemical
... a. These were discovered by Fritz London in 1930. b. They are also known as Van der Waals Interactions associated with enzymes. c. They are the temporary interactions between molecules due to temporary “clumping/dispersion” of electrons on one side of an atoms nucleus. This temporary “clumping” crea ...
... a. These were discovered by Fritz London in 1930. b. They are also known as Van der Waals Interactions associated with enzymes. c. They are the temporary interactions between molecules due to temporary “clumping/dispersion” of electrons on one side of an atoms nucleus. This temporary “clumping” crea ...
Universidad de Cantabria ON LIGHT SCATTERING BY NANOPARTICLES WITH CONVENTIONAL AND NON-CONVENTIONAL
... recent research. Several studies have concentrated on the design of nano-metamaterials composed of magnetodielectric nanoparticles forming an array [53, 54]. An important model of these arrangements of particles is the one proposed by Alù et al. [8]. They propose a structure composed of six silver n ...
... recent research. Several studies have concentrated on the design of nano-metamaterials composed of magnetodielectric nanoparticles forming an array [53, 54]. An important model of these arrangements of particles is the one proposed by Alù et al. [8]. They propose a structure composed of six silver n ...
Slide 1
... Radiative Transfer Theory • Case considered: – horizontally infinite but vertically finite plane parallel medium (air) embedded with infinitessimal oriented scattering objects at low density – canopy lies over soil surface (lower boundary) – assume horizontal homogeneity ...
... Radiative Transfer Theory • Case considered: – horizontally infinite but vertically finite plane parallel medium (air) embedded with infinitessimal oriented scattering objects at low density – canopy lies over soil surface (lower boundary) – assume horizontal homogeneity ...
[pdf]
... If absorption length la is of the order of L 2 /l* or less, this result is modified by a factor of P/sinh(p), where p is (3L2/l*la)l/2 .1 2 In the present work we ignore absorption, as 1a is of the order of a few meters, and average pathlengths, which are of the order of L 2 /l*, are always less tha ...
... If absorption length la is of the order of L 2 /l* or less, this result is modified by a factor of P/sinh(p), where p is (3L2/l*la)l/2 .1 2 In the present work we ignore absorption, as 1a is of the order of a few meters, and average pathlengths, which are of the order of L 2 /l*, are always less tha ...
AP Chemistry Summer Work
... some of you will find it to be down-right hard. There is a lot to cover and while we can do it we will all need to work very hard. You should expect this class to be SIGNIFICANTLY more difficult than your first chemistry class. This means that we cannot slow down if you don’t understand a topic. You ...
... some of you will find it to be down-right hard. There is a lot to cover and while we can do it we will all need to work very hard. You should expect this class to be SIGNIFICANTLY more difficult than your first chemistry class. This means that we cannot slow down if you don’t understand a topic. You ...
USNCO 2004 National
... pencil. Make a heavy, full mark, but no stray marks. If you decide to change an answer, erase the unwanted mark very carefully. There is only one correct answer to each question. Any questions for which more than one response has been blackened will not be counted. Your score is based solely on the ...
... pencil. Make a heavy, full mark, but no stray marks. If you decide to change an answer, erase the unwanted mark very carefully. There is only one correct answer to each question. Any questions for which more than one response has been blackened will not be counted. Your score is based solely on the ...
Advanced Atomic, Molecular and Optical Physics
... information/knowledge on atoms/molecules (the level of Experimental Physics IV: Atomic Physic): Spectroscopy of hydrogen numbers, transitions) ...
... information/knowledge on atoms/molecules (the level of Experimental Physics IV: Atomic Physic): Spectroscopy of hydrogen numbers, transitions) ...
Page 1 of 25
... c. Definite volume; shape of container; no intermolecular attractions d. Volume and shape of container; no intermolecular attractions e. Volume and shape of container; strong intermolecular attractions 102. Which transformation is evaporation? a. liquid ---> solid d. solid ---> gas b. liquid ---> ga ...
... c. Definite volume; shape of container; no intermolecular attractions d. Volume and shape of container; no intermolecular attractions e. Volume and shape of container; strong intermolecular attractions 102. Which transformation is evaporation? a. liquid ---> solid d. solid ---> gas b. liquid ---> ga ...
The influence of effective mass on magnetoresistance in ultrathin Fe/Cr/Fe films K. W
... originally discovered in Fe/Cr/Fe multilayers [1, 2]. GMR is the change of electrical resistance observed when rotating from an antiparallel to parallel alignment of film magnetizations. For its description, two different approaches are usually used: the quasi-classical method based on the Boltzmann ...
... originally discovered in Fe/Cr/Fe multilayers [1, 2]. GMR is the change of electrical resistance observed when rotating from an antiparallel to parallel alignment of film magnetizations. For its description, two different approaches are usually used: the quasi-classical method based on the Boltzmann ...
TEST on Atomic Structure
... Moving from left-to-right across a period (row) of the periodic table, 24) TRUE or FALSE - the ionization energy of the elements generally decreases 25) TRUE or FALSE - the atomic radius of the elements generally decreases NOTE: There are only 10 multiple choice questions on the test – MOST are SIM ...
... Moving from left-to-right across a period (row) of the periodic table, 24) TRUE or FALSE - the ionization energy of the elements generally decreases 25) TRUE or FALSE - the atomic radius of the elements generally decreases NOTE: There are only 10 multiple choice questions on the test – MOST are SIM ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.












![[pdf]](http://s1.studyres.com/store/data/008852311_1-a80c01e7dd06bde7495e825ae8833165-300x300.png)









