Lec. 42 notes
... C. A quantum system may not have a precisely defined energy D. More than one of the above E. None of the above A. The energy of a particle is only quantized when the particle is confined (infinite square well, finite square well, atom, simple harmonic oscillator). It comes from boundary condition ...
... C. A quantum system may not have a precisely defined energy D. More than one of the above E. None of the above A. The energy of a particle is only quantized when the particle is confined (infinite square well, finite square well, atom, simple harmonic oscillator). It comes from boundary condition ...
The Title Goes Here
... Laser-induced breakdown spectroscopy is a wellknown technique for rapid, in situ analysis of materials. It is a promising approach for standoff detection of potentially hazardous or difficult to access nuclear materials. LIBS employs an intense laser pulse to generate a plasma on the surface of a ta ...
... Laser-induced breakdown spectroscopy is a wellknown technique for rapid, in situ analysis of materials. It is a promising approach for standoff detection of potentially hazardous or difficult to access nuclear materials. LIBS employs an intense laser pulse to generate a plasma on the surface of a ta ...
Magneto Optical Kerr Effect (MOKE)
... parent bulk material, such as its crystalline structure, but also on the shape, size and thickness of the nano-elements. Therefore the anisotropy of a nano-structure can be controlled using its geometry, or shape. The angular dependence of the total energy of the nanostructure in the uniform magneti ...
... parent bulk material, such as its crystalline structure, but also on the shape, size and thickness of the nano-elements. Therefore the anisotropy of a nano-structure can be controlled using its geometry, or shape. The angular dependence of the total energy of the nanostructure in the uniform magneti ...
1 Introduction - High Point University
... Because the states an electron occur only at discrete energy levels, they are said to be quantized. The word quantum comes from a Latin word meaning “how much.” The branch of physics that provides the current model of the Hydrogen atom is called quantum mechanics. The electron in a Hydrogen atom can ...
... Because the states an electron occur only at discrete energy levels, they are said to be quantized. The word quantum comes from a Latin word meaning “how much.” The branch of physics that provides the current model of the Hydrogen atom is called quantum mechanics. The electron in a Hydrogen atom can ...
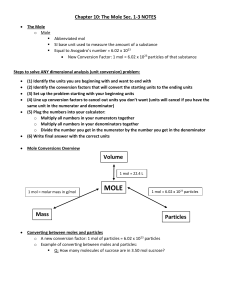
MOLE Mass Volume Particles
... SI base unit used to measure the amount of a substance Equal to Avogadro’s number = 6.02 x 1023 New Conversion Factor: 1 mol = 6.02 x 1023 particles of that substance ...
... SI base unit used to measure the amount of a substance Equal to Avogadro’s number = 6.02 x 1023 New Conversion Factor: 1 mol = 6.02 x 1023 particles of that substance ...
Compulsory textbook Recommended textbooks Topics of the first
... analyte with accurately known composition (or stoichiometry) • Drying: t < 200 oC • Ignition: t = 6-800 oC • If the filtration is done with filter paper, ignition can be done, if glass filter is used, only drying is allowed • Weighing is always done by using an analytical balance • The weighed mass ...
... analyte with accurately known composition (or stoichiometry) • Drying: t < 200 oC • Ignition: t = 6-800 oC • If the filtration is done with filter paper, ignition can be done, if glass filter is used, only drying is allowed • Weighing is always done by using an analytical balance • The weighed mass ...
Chemistry Entrance Material for Grade 11 to 12
... Based on this data alone, which of the above substances is expected to have the highest molar heat of fusion? 31. The temperature at the boiling point or melting point stays the same). ...
... Based on this data alone, which of the above substances is expected to have the highest molar heat of fusion? 31. The temperature at the boiling point or melting point stays the same). ...
How many grams of oxygen are made if 3.75 moles of KClO 3
... garlic that contains no more than 6.89 mol of allyl sulfide. You were hired by Cedar as a chemistry consultant to calculate the maximum mass of allyl sulfide that should be included in the recipe for the hummus to turn out scrumptious. What kind of feedback would you give them? How many grams would ...
... garlic that contains no more than 6.89 mol of allyl sulfide. You were hired by Cedar as a chemistry consultant to calculate the maximum mass of allyl sulfide that should be included in the recipe for the hummus to turn out scrumptious. What kind of feedback would you give them? How many grams would ...
mark scheme - A-Level Chemistry
... Multiply m/z by relative abundance for each isotope (1) Allow instead of m/z mass no, Ar or actual value from example Sum these values (1) Divide by the sum of the relative abundances (1) only award this mark if previous 2 given Max 2 if e.g. has only 2 isotopes ...
... Multiply m/z by relative abundance for each isotope (1) Allow instead of m/z mass no, Ar or actual value from example Sum these values (1) Divide by the sum of the relative abundances (1) only award this mark if previous 2 given Max 2 if e.g. has only 2 isotopes ...
exploiting the superposition principle foundations and applications
... (all tools readily available, see also J. Close experiments) • First matter wave interferences with a dilute coherent atomic wave packet (nK energy) during extended free fall • DKC makes it possible to venture towards energies in the ...
... (all tools readily available, see also J. Close experiments) • First matter wave interferences with a dilute coherent atomic wave packet (nK energy) during extended free fall • DKC makes it possible to venture towards energies in the ...
Atomic Theory - Relativistic quantum dynamics of ions and beams
... Interactions of light with atoms and matter: ➣ Light can also undergo reflection, scattering and absorption; sometimes, the energy/heat transfer through a material is mostly radiative, i.e. by emission and absorption of photons (for example, in the core of the Sun). ➣ Light of different frequencies ma ...
... Interactions of light with atoms and matter: ➣ Light can also undergo reflection, scattering and absorption; sometimes, the energy/heat transfer through a material is mostly radiative, i.e. by emission and absorption of photons (for example, in the core of the Sun). ➣ Light of different frequencies ma ...
Chapters 1-3 Packet
... Homework: You will be given an assignment sheet at the beginning of each unit which lists your homework. You will have homework (almost) every night in this class. It is strongly recommended that you do all of the problems, but your grade will be a result of your performance on the quiz that covers ...
... Homework: You will be given an assignment sheet at the beginning of each unit which lists your homework. You will have homework (almost) every night in this class. It is strongly recommended that you do all of the problems, but your grade will be a result of your performance on the quiz that covers ...
Chapter 4
... BaCI2 (aq) + Na2SO4 (aq) Ba2+(aq) + 2Cl-(aq) + 2Na+(aq)+ SO4 2-(aq) 2. Match cation from one salt with the anion from the other salt” Ba2+(aq) + Cl-(aq) + Na+(aq)+ SO4 2-(aq) NaCl+ BaSO4 Note: Always keep the metal on the left in all salts! 3. Balance charges in salts and put in coefficients Ba2+( ...
... BaCI2 (aq) + Na2SO4 (aq) Ba2+(aq) + 2Cl-(aq) + 2Na+(aq)+ SO4 2-(aq) 2. Match cation from one salt with the anion from the other salt” Ba2+(aq) + Cl-(aq) + Na+(aq)+ SO4 2-(aq) NaCl+ BaSO4 Note: Always keep the metal on the left in all salts! 3. Balance charges in salts and put in coefficients Ba2+( ...
stoichiometry power point File
... 3.1 Counting by Weighing • If the average mass of an amount of particles is taken particles behave as though they were all identical for the purposes of weighing (refer to the jelly bean example on p. 77-78). • Since atoms are small it makes more sense to count them by mass than by getting out our ...
... 3.1 Counting by Weighing • If the average mass of an amount of particles is taken particles behave as though they were all identical for the purposes of weighing (refer to the jelly bean example on p. 77-78). • Since atoms are small it makes more sense to count them by mass than by getting out our ...
Unit 8 Note Packet
... Enthalpy: a measure of the energy content of a substance. This includes kinetic and potential. (It also includes pressure-volume energy but we will ignore this in general chemistry). Since we will always be dealing with constant pressure systems in chemistry (until we get to the gas laws unit), we c ...
... Enthalpy: a measure of the energy content of a substance. This includes kinetic and potential. (It also includes pressure-volume energy but we will ignore this in general chemistry). Since we will always be dealing with constant pressure systems in chemistry (until we get to the gas laws unit), we c ...
Spectra of Underwater Light-Field Fluctuations in the Photic Zone
... This is in good agreement with the appearance of the sharp peak occurring in the 4-meter spectrum. There are, of course, other benefits to this kind of analysis of irradiance data which do not contribute to the biological application discussed above, but should be briefly discussed for the sake of c ...
... This is in good agreement with the appearance of the sharp peak occurring in the 4-meter spectrum. There are, of course, other benefits to this kind of analysis of irradiance data which do not contribute to the biological application discussed above, but should be briefly discussed for the sake of c ...
Optical Properties of Silica-Copper Oxide Thin Films Prepared by Spin Coating
... wavelength region 250-1700 nm at three different incident angles, 60°, 65° and 70°. Measurements were also performed in the same wavelength region and with the same incident angles on a substrate with no film, i.e. with only native oxide present. This was to obtain the thickness of the native oxide ...
... wavelength region 250-1700 nm at three different incident angles, 60°, 65° and 70°. Measurements were also performed in the same wavelength region and with the same incident angles on a substrate with no film, i.e. with only native oxide present. This was to obtain the thickness of the native oxide ...
A Review of High School Chemistry
... Acid Base Chemistry What is the big deal with acids and bases? Again, it is because we are working with water, which, as we will see, has its own chemistry that produces ions like H+ and OH-, and which as everyone knows, are what Arrhenius called acids and bases. Consequently, we will spend a lot of ...
... Acid Base Chemistry What is the big deal with acids and bases? Again, it is because we are working with water, which, as we will see, has its own chemistry that produces ions like H+ and OH-, and which as everyone knows, are what Arrhenius called acids and bases. Consequently, we will spend a lot of ...
Final Exam Practice Problems: R = 0.0821 Latm/molK NA = 6.022
... A) Li+ (aq) + SO42-(aq) + Cu+(aq) + NO3-(aq) → CuS(s) + Li+(aq) + NO3-(aq) B) Li+ (aq) + S-(aq) + Cu+(aq) + NO3-(aq) → CuS(s) + LiNO3(aq) C) 2 Li+(aq) + S2-(aq) + Cu2+(aq) + 2 NO3-(aq) → Cu2+(aq) + S2-(aq) + 2 LiNO3(s) D) 2 Li+(aq) + S2-(aq) + Cu2+(aq) + 2 NO3-(aq) → CuS(s) + 2 Li+(aq) + 2 NO3-(aq) ...
... A) Li+ (aq) + SO42-(aq) + Cu+(aq) + NO3-(aq) → CuS(s) + Li+(aq) + NO3-(aq) B) Li+ (aq) + S-(aq) + Cu+(aq) + NO3-(aq) → CuS(s) + LiNO3(aq) C) 2 Li+(aq) + S2-(aq) + Cu2+(aq) + 2 NO3-(aq) → Cu2+(aq) + S2-(aq) + 2 LiNO3(s) D) 2 Li+(aq) + S2-(aq) + Cu2+(aq) + 2 NO3-(aq) → CuS(s) + 2 Li+(aq) + 2 NO3-(aq) ...
Band Theories
... If the atomic p orbitals lie higher in energy than the s orbitals, the the p band lies higher in energy than the s band and there may be a band gap – a range of energies to which no orbital corresponds. ...
... If the atomic p orbitals lie higher in energy than the s orbitals, the the p band lies higher in energy than the s band and there may be a band gap – a range of energies to which no orbital corresponds. ...
Balance this equation:
... The diagram shows iron oxide, Fe2O3, and carbon monoxide, CO reacting to form iron and carbon dioxide. Which of the following is the correct full balanced chemical equation for the reaction depicted? ...
... The diagram shows iron oxide, Fe2O3, and carbon monoxide, CO reacting to form iron and carbon dioxide. Which of the following is the correct full balanced chemical equation for the reaction depicted? ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.