* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download stoichiometry power point File
Drug discovery wikipedia , lookup
Rate equation wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Chemical element wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
History of molecular theory wikipedia , lookup
Mass spectrometry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Isotopic labeling wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
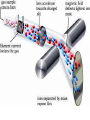
3.1 Counting by Weighing • If the average mass of an amount of particles is taken particles behave as though they were all identical for the purposes of weighing (refer to the jelly bean example on p. 77-78). • Since atoms are small it makes more sense to count them by mass than by getting out our micro-tweezers. 3.2 Atomic Masses • All masses are relative to 12C. This isotope of carbon was assigned a mass of 12 atomic mass units. • The most accurate method of weighing atoms is with a mass spectrometer. – Uses magnets to deflect ions. – Heavier ions are deflected less. • Recall that the atomic mass on the periodic table is a weighted average of all known isotopes. 3.2 Atomic Masses • To find the average atomic mass for a given element the mass of each isotope is multiplied by its relative abundance. The products of each known isotope are then added together to give the average atomic mass. • Here’s an example…. 3.2 Atomic Masses There are 2 naturally occurring isotopes of Cu Cu-63 62.93 amu 69.09 % abundance Cu-65 64.93 amu 30.91 % abundance (.6909 x 62.93) + (.3091 x 64.93) = 63.55 amu 3.3 The Mole • The mole is the number of unbound carbon atoms found in 12 grams of 12C. – This value is 6.022 x 1023 units or entities. • The mole is defined such that a sample of a natural element with a mass equal to its atomic mass will contain 1 mole of atoms. 3.4 Molar Mass • Molar mass is the mass in grams of one mole of a compound. • This is found by added up the atomic masses of all atoms present in a molecule. • CH4 = 16 g / mol 3.5 Solving Problems • Identify ending point • Identify starting point • Choose an efficient route to get there (riding the mole train) --- preferably using dimensional analysis • Check --- does your answer make sense? • Check --- are units and sig figs correct? (see back of mole train guide for more detail) 3.6 % Composition of Cmpds. • We can either describe a compound by listing the number of the atoms that make it up (the formula) or by a list of atomic percentages (usually from lab data). • To find the percentage one take the total mass of an element from a compound and divides it by the total mass of the compound. • Here’s an example…. 3.6 % Composition of Cmpds Find the % composition (by mass) of H2O H: 2 mol x 1 g/mol = 2 g O: 1 mol x 16 g/mol = 16 g Molar Mass of H2O = 18 g H: 2/18 x 100 = 11.1% O: 16/18 x 100 = 89.9% 3.7 Formula Determination • Rarely, if ever, do lab results yield the exact formula for an unknown compounds. • Instead mass data or ratio data is used to find the formula for the unknown compound. • You may be given the masses as percentages (15% of O, 85% C) or you may be given masses of the products. • Here’s an example…about empirical and molecular formulas. 3.7 Formula Determination A compound contains 42 g N and 6.0 g H Molar Mass = 32 g/mol Find empirical and molecular formula N: 42 g x 1 mol / 14 g = 3.0 mol H: 6 g x 1 mol / 1.0 g = 6.0 mol Empirical formula is NH2 3.0/3.0 = 1 6.0/3.0 = 2 3.7 Formula Determination Molecular form = emp form x (actual molar mass / molar mass of emp form) NH2 x 32/16 = N2H4 3.8 Chemical Equations • All atoms must be accounted for on both sides of the reaction arrow (in other words, BALANCE THE EQUATION). • This is mainly guess and check, but here are some tips: – Balance the biggest compounds first. – Balance odd coefficients. – Leave the smallest compounds (O2, H2O) for last. 3.8 Chemical Equations Predicting products (some hints): • Metal hydroxide metal oxide + water Zn(OH)2 ZnO + H20 • Metal chlorate metal chloride + oxygen 2 KClO3 2 KCl + 3 O2 • Metal carbonate metal oxide + carbon dioxide K2CO3 K2O + CO2 3.8 Chemical Equations Predicting products (some hints continued): • Decomposition of acids with S,N H2SO4 H2O + SO3 • Alkali metal + water metal hydroxide + hydrogen 2 Na + 2 H2O 2 NaOH + H2 • Hydrocarbon + oxygen carbon dioxide + water CH4 + 2 O2 CO2 + 2 H20 3.10 Stoichiometric Calculations • Once a chemical equation is balanced we can use the masses of the reactants to determine the masses of the products. • The number of molecules in a given mass of a compound is equal to the number of molecules of a different compound with an equal mass. • So we must convert the masses given to moles. 3.10 Stoich. Calculations • Using mole ratios we can relate the given moles of products and reactants. What mass of water vapor forms when 32 grams of methane undergoes complete combustion? CH4 + 2 O2 CO2 + 2 H20 32 g CH4 x (1 mol CH4 / 16 g CH4) x (2 mol H20 / 1 mol CH4) x (18 g H2O / 1 mol H2O) = 72 g H2O 3.10 Stoich. Calculations • Steps to finding masses in a chemical reaction: – Balance the reaction. – Convert the known mass to moles. – Determine the mole ratio between what is given and what is asked for. – Convert back to grams if necessary. 3.11 Limiting Reagent • Considering the following Recipe: 6 strips of bacon + 6 leaves of lettuce + 4 tomato slices + 2 slices of toast 3.11 Limiting Reagent • So given the following ingredients how many of the GREATEST B.L.T. EVER can be made?? – 20 slices of bacon – 43 leaves of lettuce – 45 tomato slices – 160 slices of toast. 3.11 Limiting Reagent • Convert the masses of all reactants to moles. • Compare moles to determine the LR and XS reactants • LR gets used up so … – Use LR to determine amount of products formed – Use LR to determine amount of XS reactants left More details on stoichiometry guide 3.11 Limiting Reagent 100 grams of hydrogen gas 720 grams of oxygen gas react as much as possible to form water vapor. Write a balanced equation for the reaction. What is the limiting reactant? What reactant is in XS? How much of it is left over? What mass of water vapor is produced