Lecture 30/3 Nuclear processes Ulf Torkelsson 1 Nuclear reactions
... nucleon decreases with the size of the nucleus. Therefore there are two ways of deriving energy from atomic nuclei, by fusing light nuclei, and by fissioning heavy nuclei. The nuclei that are involved in a fusion reaction are repelling each other through the Coulomb force, since they are both positi ...
... nucleon decreases with the size of the nucleus. Therefore there are two ways of deriving energy from atomic nuclei, by fusing light nuclei, and by fissioning heavy nuclei. The nuclei that are involved in a fusion reaction are repelling each other through the Coulomb force, since they are both positi ...
Nuclear Reactions
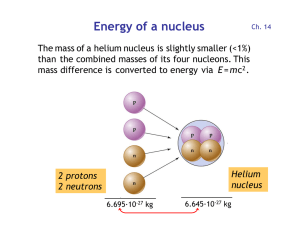
... The missing mass (mass defect) has been stored as energy in the nucleus. It is called the binding energy of the nucleus. ...
... The missing mass (mass defect) has been stored as energy in the nucleus. It is called the binding energy of the nucleus. ...
Unit #12: Nuclear Chemistry
... DON’T COPY: Radioactive C-14 is formed in the upper atmosphere by nuclear reactions initiated by neutrons in cosmic radiation 14N + 1 n ---> 14C + 1H ...
... DON’T COPY: Radioactive C-14 is formed in the upper atmosphere by nuclear reactions initiated by neutrons in cosmic radiation 14N + 1 n ---> 14C + 1H ...
Fission and Fusion
... In this example, a stray neutron strikes an atom of U-235. It absorbs the neutron and becomes an unstable atom of U-236. It then undergoes fission. Notice that more neutrons are released in the reaction. These neutrons can strike other U-235 atoms to initiate their fission. ...
... In this example, a stray neutron strikes an atom of U-235. It absorbs the neutron and becomes an unstable atom of U-236. It then undergoes fission. Notice that more neutrons are released in the reaction. These neutrons can strike other U-235 atoms to initiate their fission. ...
Fusion or Fission
... get it started. In the right conditions, it can sustain itself, once started. One of the benefits of fusion reactions is that there are no radioactive byproducts. On earth, fusion reactions result in the element helium. Heavier elements, like beryllium or carbon, are produced by fusion in stars. Hyd ...
... get it started. In the right conditions, it can sustain itself, once started. One of the benefits of fusion reactions is that there are no radioactive byproducts. On earth, fusion reactions result in the element helium. Heavier elements, like beryllium or carbon, are produced by fusion in stars. Hyd ...
Atom and Nucleus. Radioactivity. Nuclear Energy.
... Ernest Rutherford attempted to test this model by bombarding a thin gold foil with alpha-particles. A significant scattering of the alpha-particles was ...
... Ernest Rutherford attempted to test this model by bombarding a thin gold foil with alpha-particles. A significant scattering of the alpha-particles was ...
Nuclear Reactions Review
... 3.Nuclei with too many or too few neutrons are A.never found. C.unnatural. B.unstable. D.stable. ...
... 3.Nuclei with too many or too few neutrons are A.never found. C.unnatural. B.unstable. D.stable. ...
Nuclear Reactions Review powerpt
... 3.Nuclei with too many or too few neutrons are A.never found. C.unnatural. B.unstable. D.stable. ...
... 3.Nuclei with too many or too few neutrons are A.never found. C.unnatural. B.unstable. D.stable. ...
Nuclear Fission and Fusion
... 1) If a radioactive sample of rock started with 100 counts per second and 2 hours later has a count rate of 25 counts per second, what is it’s half-life? Try these questions, have a go at the answer 1) What do you think is meant by a ‘chain reaction?’ 2) The gold that exists on our planet, where did ...
... 1) If a radioactive sample of rock started with 100 counts per second and 2 hours later has a count rate of 25 counts per second, what is it’s half-life? Try these questions, have a go at the answer 1) What do you think is meant by a ‘chain reaction?’ 2) The gold that exists on our planet, where did ...
Document
... 1. The amount of material left after two half-lives is _one-fourth (1/4) _ of the original amount. 2. _Fission_ means "to divide." 3. _Nuclear Fusion_ is the combining of two low-mass nuclei into one nucleus with a larger mass. 4. Radioactive isotopes that are put into the body to monitor a bodily p ...
... 1. The amount of material left after two half-lives is _one-fourth (1/4) _ of the original amount. 2. _Fission_ means "to divide." 3. _Nuclear Fusion_ is the combining of two low-mass nuclei into one nucleus with a larger mass. 4. Radioactive isotopes that are put into the body to monitor a bodily p ...
1.6--NOTES--Detecting Radiation Nuclear Rxtns
... The nuclear reaction that splits large nuclei into 2 smaller nuclei is (fission, fusion). ...
... The nuclear reaction that splits large nuclei into 2 smaller nuclei is (fission, fusion). ...
Fission and Fusion
... • In controlled fission water or graphite are used to absorb some of the neutrons produced by fission, controlling the rate of the fission process. • Fusion is the fusing of two unstable hydrogen atoms to form helium. • Fusion is harder to start and control than fission, but produces more energy per ...
... • In controlled fission water or graphite are used to absorb some of the neutrons produced by fission, controlling the rate of the fission process. • Fusion is the fusing of two unstable hydrogen atoms to form helium. • Fusion is harder to start and control than fission, but produces more energy per ...