Nuclear Reactions - Socastee High School
... How To Make an H-Bomb • Unlike fission bombs, which rely only on nuclear fission, and which can achieve explosions equivalent to thousands of tons of TNT ("kilotons"), the power of an H-bomb or thermonuclear weapon has no practical limit -it can be made as powerful as you want, by adding more deute ...
... How To Make an H-Bomb • Unlike fission bombs, which rely only on nuclear fission, and which can achieve explosions equivalent to thousands of tons of TNT ("kilotons"), the power of an H-bomb or thermonuclear weapon has no practical limit -it can be made as powerful as you want, by adding more deute ...
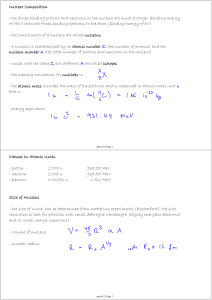
Masses in Atomic Units - proton 1.007 u 938.28 MeV
... - small nuclei can lower their energy by forming larger nuclei in a process called fusion - large nuclei can lower their energy by breaking up into smaller nuclei in a process called fission - the most stable nucleus is an isotope of iron (Fe) - the binding energy of nucleons is extremely large comp ...
... - small nuclei can lower their energy by forming larger nuclei in a process called fusion - large nuclei can lower their energy by breaking up into smaller nuclei in a process called fission - the most stable nucleus is an isotope of iron (Fe) - the binding energy of nucleons is extremely large comp ...
6.2 Atomic Nucleus Stability and Isotopes
... positron generated by decay is quickly annihilated by the relative excess of electrons. ...
... positron generated by decay is quickly annihilated by the relative excess of electrons. ...
Nuclear reactions: fission and fusion
... (elements with low atomic numbers). In a hydrogen bomb, two isotopes of hydrogen, deuterium and tritium are fused to form a nucleus of helium and a neutron. This fusion releases 17.6 MeV of energy. Unlike nuclear fission, there is no limit on the amount of the fusion that can occur. The immense ener ...
... (elements with low atomic numbers). In a hydrogen bomb, two isotopes of hydrogen, deuterium and tritium are fused to form a nucleus of helium and a neutron. This fusion releases 17.6 MeV of energy. Unlike nuclear fission, there is no limit on the amount of the fusion that can occur. The immense ener ...
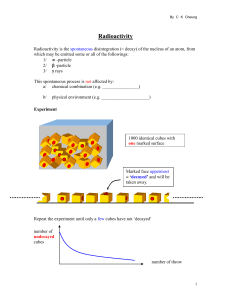
Radioactivity
... minute per gram of carbon. But when the organism dies no intake of C*, but decay process goes on with T1/2 ~ 5700 years. By measuring the activity of some furniture from an ancient village age of the furniture can be estimated within the range of 1000 to 5000 years. ...
... minute per gram of carbon. But when the organism dies no intake of C*, but decay process goes on with T1/2 ~ 5700 years. By measuring the activity of some furniture from an ancient village age of the furniture can be estimated within the range of 1000 to 5000 years. ...
- Physics
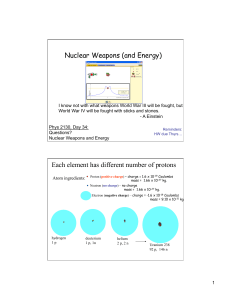
... An uncontrolled chain reaction can occur if enough uranium-235 nuclei are in close proximity to each other. If the uranium-235 is in the shape of a sphere about 13 pounds of uranium form a critical mass where a runaway chain reaction (bomb) can occur. I would restate the last paragraph on page 20-4. ...
... An uncontrolled chain reaction can occur if enough uranium-235 nuclei are in close proximity to each other. If the uranium-235 is in the shape of a sphere about 13 pounds of uranium form a critical mass where a runaway chain reaction (bomb) can occur. I would restate the last paragraph on page 20-4. ...
Nuclear Chemistry
... of matter is upheld. The mass numbers & atomic numbers should add up to be equal on both sides of the equation ...
... of matter is upheld. The mass numbers & atomic numbers should add up to be equal on both sides of the equation ...
25.3 Fission and Fusion of Atomic Nuclei
... The sun, directly and indirectly, is the source of most energy used on Earth. The energy released by the sun results from nuclear fusion. Fusion occurs when nuclei combine to produce a nucleus of greater mass. In solar fusion, hydrogen nuclei (protons) fuse to make helium nuclei. Figure 25.13 shows ...
... The sun, directly and indirectly, is the source of most energy used on Earth. The energy released by the sun results from nuclear fusion. Fusion occurs when nuclei combine to produce a nucleus of greater mass. In solar fusion, hydrogen nuclei (protons) fuse to make helium nuclei. Figure 25.13 shows ...
CHAPTER 13: Nuclear Interactions and Applications
... The factor Q is used to represent the ratio of the power produced in the fusion reaction to the power required to produce the fusion (heat). This Q factor is not to be confused with the Q value. The breakeven point is Q = 1, and ignition occurs for Q >> 1. For controlled fusion produced in the labor ...
... The factor Q is used to represent the ratio of the power produced in the fusion reaction to the power required to produce the fusion (heat). This Q factor is not to be confused with the Q value. The breakeven point is Q = 1, and ignition occurs for Q >> 1. For controlled fusion produced in the labor ...
Chapter 7 Worksheet
... which can cause three additional fission reactions and start a large chain reaction of fission reactions. C Kypton-92 and barium-141 are unstable products. These products can further decay into other isotopes releasing more useful energy. ...
... which can cause three additional fission reactions and start a large chain reaction of fission reactions. C Kypton-92 and barium-141 are unstable products. These products can further decay into other isotopes releasing more useful energy. ...
35Nuclear.old
... B. Hard because protons are repelled from each other by the electromagnetic force C. Easy because protons are attracted to each other by the force of gravity D. Hard because protons are repelled from each other by the force of gravity ...
... B. Hard because protons are repelled from each other by the electromagnetic force C. Easy because protons are attracted to each other by the force of gravity D. Hard because protons are repelled from each other by the force of gravity ...
Nuclear chemistry – the study of nuclear reactions and their uses in
... Nuclear power plants and nuclear weapons depend on fission. When heavy nuclei split due to the capture of neutrons, radioactive isotopes of many different elements are formed. If one fission produces 2 neutrons, these 2 neutrons can produce four fissions, and so forth. i. Reactions that multiple in ...
... Nuclear power plants and nuclear weapons depend on fission. When heavy nuclei split due to the capture of neutrons, radioactive isotopes of many different elements are formed. If one fission produces 2 neutrons, these 2 neutrons can produce four fissions, and so forth. i. Reactions that multiple in ...