FUSION AND FISSION
... • Alpha, beta, and gamma • Nuclear reactions involve decay occur as ONE more than just getting rid of atom tries to increase a few protons or neutrons. it’s stability by getting rid The new atoms produced of a few neutrons, or are VERY different protons & neutrons. elements than the reactant. • The ...
... • Alpha, beta, and gamma • Nuclear reactions involve decay occur as ONE more than just getting rid of atom tries to increase a few protons or neutrons. it’s stability by getting rid The new atoms produced of a few neutrons, or are VERY different protons & neutrons. elements than the reactant. • The ...
Chapter 7 - Bakersfield College
... charged lumps of matter with electrons embedded in them. B. In 1911, an experiment suggested by British physicist Ernest Rutherford shows that alpha particles striking a thin metal foil are deflected by the strong electric fields of the metal atom's nuclei. C. Rutherford's experiment resulted in the ...
... charged lumps of matter with electrons embedded in them. B. In 1911, an experiment suggested by British physicist Ernest Rutherford shows that alpha particles striking a thin metal foil are deflected by the strong electric fields of the metal atom's nuclei. C. Rutherford's experiment resulted in the ...
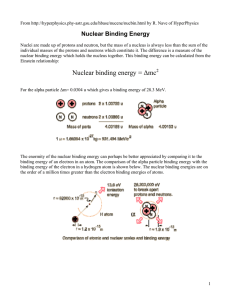
Nuclear binding energy = Δmc2 - University of Toronto Physics
... Nuclear Binding Energy Curve The binding energy curve is obtained by dividing the total nuclear binding energy by the number of nucleons. The fact that there is a peak in the binding energy curve in the region of stability near iron means that either the breakup of heavier nuclei (fission) or the co ...
... Nuclear Binding Energy Curve The binding energy curve is obtained by dividing the total nuclear binding energy by the number of nucleons. The fact that there is a peak in the binding energy curve in the region of stability near iron means that either the breakup of heavier nuclei (fission) or the co ...
Mass-Energy Equivalence - Dr. Haleys Physics Class
... four new nuclei and cause fission reactions that release eight neutrons. The number of neutrons increases rapidly. The increasing number of neutrons causes more nuclei to have fission reactions and enormous energy is released. ...
... four new nuclei and cause fission reactions that release eight neutrons. The number of neutrons increases rapidly. The increasing number of neutrons causes more nuclei to have fission reactions and enormous energy is released. ...
Ch 10 Nuclear Chemistry
... atomic nucleus emits charged particles and energy. • Radioisotope is short for radioactive isotopes, which is any atom containing an unstable nucleus. • Radioisotopes spontaneously change into other isotopes over time and is said to undergo nuclear ...
... atomic nucleus emits charged particles and energy. • Radioisotope is short for radioactive isotopes, which is any atom containing an unstable nucleus. • Radioisotopes spontaneously change into other isotopes over time and is said to undergo nuclear ...
AC Geophysical Science Study Guide
... 11. Which element(s) has the highest proton to neutron ratio? What is that ratio? 12. Which element(s) has the lowest proton to neutron ratio? What is that ratio? 13. Which element(s) has the lowest proton to electron ratio? The highest ratio? What is the ratio? 14. Describe the strong force. What r ...
... 11. Which element(s) has the highest proton to neutron ratio? What is that ratio? 12. Which element(s) has the lowest proton to neutron ratio? What is that ratio? 13. Which element(s) has the lowest proton to electron ratio? The highest ratio? What is the ratio? 14. Describe the strong force. What r ...
Nuclear Decay
... amount of energy gets released at extremely high temperatures: nearly 150 million degrees Celsius. At extreme temperatures, electrons are separated from nuclei and a gas becomes a plasma—a hot, electrically charged gas. ...
... amount of energy gets released at extremely high temperatures: nearly 150 million degrees Celsius. At extreme temperatures, electrons are separated from nuclei and a gas becomes a plasma—a hot, electrically charged gas. ...
Radioactivity - Mrs. Sjuts` Science Site
... When an unstable nucleus decays, particles and energy are emitted from the decaying nucleus Alpha Particles – (2 p and 2 n lost) massive, comparatively speaking; loses energy quickly; can’t pass through paper; changes the element (transmutation); mass changes; can damage the body Beta Particles – (n ...
... When an unstable nucleus decays, particles and energy are emitted from the decaying nucleus Alpha Particles – (2 p and 2 n lost) massive, comparatively speaking; loses energy quickly; can’t pass through paper; changes the element (transmutation); mass changes; can damage the body Beta Particles – (n ...
Energy per nucleon
... • All elements heavier than iron/nickel are created during a supernova explosion, which has enough thermal energy to form nuclei with higher energy per nucleon. ...
... • All elements heavier than iron/nickel are created during a supernova explosion, which has enough thermal energy to form nuclei with higher energy per nucleon. ...