Atomic Spectroscopy With Reference To The Textbook Atomic
... The First Balmer Series line Ha Wavelength of the n = 3 - 2 transition: 6563 Angstroms RED COLOR ...
... The First Balmer Series line Ha Wavelength of the n = 3 - 2 transition: 6563 Angstroms RED COLOR ...
Supplementary Information Dynamically Tuning the Up
... number, S is the spin quantum number. PJ is the total angular momentum, is the total magnetic moment. J is the effect total magnetic moment to total angular momentum. ...
... number, S is the spin quantum number. PJ is the total angular momentum, is the total magnetic moment. J is the effect total magnetic moment to total angular momentum. ...
Optical Properties of Condensed Matters
... Semiclassical: apply quantum mechanics to atoms, treat light as a classical electromagnetic wave; Fully quantum: both atoms and light are treated quantum mechanically. ...
... Semiclassical: apply quantum mechanics to atoms, treat light as a classical electromagnetic wave; Fully quantum: both atoms and light are treated quantum mechanically. ...
Chapter 2 Classical propagation
... 3. IR absorption is caused by the vibrational quanta in SiO2 molecules themselves(1.4 1013 Hz (21m) and 3.3 1013 Hz(9.1 m); 4. UV absorption is caused by interband electronic transition(band gap of about 10 eV), threshold at 2 1015 Hz(150 nm)( ~ 108 m-1); 5. UV absorption departure from Lor ...
... 3. IR absorption is caused by the vibrational quanta in SiO2 molecules themselves(1.4 1013 Hz (21m) and 3.3 1013 Hz(9.1 m); 4. UV absorption is caused by interband electronic transition(band gap of about 10 eV), threshold at 2 1015 Hz(150 nm)( ~ 108 m-1); 5. UV absorption departure from Lor ...
The Development of a New Atomic Model
... • Albert Einstein expanded on Planck’s work and proposed a radical idea • Electromagnetic radiation has a dual waveparticle nature • While like light act like a wave • It is a stream of particles • Each particle carries a quantum of energy • These particles are called photons – These particles have ...
... • Albert Einstein expanded on Planck’s work and proposed a radical idea • Electromagnetic radiation has a dual waveparticle nature • While like light act like a wave • It is a stream of particles • Each particle carries a quantum of energy • These particles are called photons – These particles have ...
Laser Physics I
... Our everyday experience of "light" generally encompasses only the small part of the electromagnetic spectrum to which the human eye is sensitive, a wavelength range running roughly from 400 nm to 700 nm. The full electromagnetic spectrum, going from low to high frequencies, is divided into radiowa ...
... Our everyday experience of "light" generally encompasses only the small part of the electromagnetic spectrum to which the human eye is sensitive, a wavelength range running roughly from 400 nm to 700 nm. The full electromagnetic spectrum, going from low to high frequencies, is divided into radiowa ...
Chapter 7: Quantum Mechanical Model of Atom
... light. Observed as white light. – Line spectrum contains specific wavelengths that result in a unique “fingerprint” that is used to identify elements. No two elements have the exact same line spectrum. ...
... light. Observed as white light. – Line spectrum contains specific wavelengths that result in a unique “fingerprint” that is used to identify elements. No two elements have the exact same line spectrum. ...
Atomic Absorption Spectrometry
... methods are used to determine the concentration of an element in solution. Both methods use a standard curve. Difference between UV and IR spectroscopy is that sample must be atomised where the analyte are converted into free atom. This is called the atomization concept. ...
... methods are used to determine the concentration of an element in solution. Both methods use a standard curve. Difference between UV and IR spectroscopy is that sample must be atomised where the analyte are converted into free atom. This is called the atomization concept. ...
Optical Properties of Condensed Matters
... Semiclassical: apply quantum mechanics to atoms, treat light as a classical electromagnetic wave; Fully quantum: both atoms and light are treated quantum mechanically. ...
... Semiclassical: apply quantum mechanics to atoms, treat light as a classical electromagnetic wave; Fully quantum: both atoms and light are treated quantum mechanically. ...
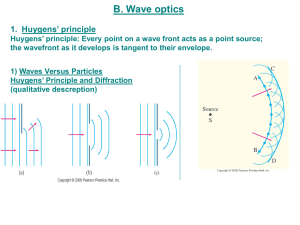
Topic 4 Waves - MrSimonPorter
... Transverse wave – the oscillations are perpendicular to the direction of energy transfer. Sporter011 ...
... Transverse wave – the oscillations are perpendicular to the direction of energy transfer. Sporter011 ...