High Resolution Biomedical Imaging with Light and Sound
... University of Michigan in 2002, where his research in the Biomedical Ultrasonics Laboratory explored optical techniques to generate and receive high frequency ultrasound. He then joined the Center for Ultrafast Optical Science at the University of Michigan to perform research in time-domain terahert ...
... University of Michigan in 2002, where his research in the Biomedical Ultrasonics Laboratory explored optical techniques to generate and receive high frequency ultrasound. He then joined the Center for Ultrafast Optical Science at the University of Michigan to perform research in time-domain terahert ...
atom and valence electron signals – x-rays – gamma-rays
... spectrum is typically used to determine the identity and quantity of gamma emitters present in a gamma source, and is a vital tool in radiometric assay. The gamma spectrum is characteristic of the gammaemitting nuclides contained in the source, just as in optical spectroscopy, the optical spectrum i ...
... spectrum is typically used to determine the identity and quantity of gamma emitters present in a gamma source, and is a vital tool in radiometric assay. The gamma spectrum is characteristic of the gammaemitting nuclides contained in the source, just as in optical spectroscopy, the optical spectrum i ...
Electro-Optic Ceramics
... (a) Linearly polarized light with Eyo = 2Exo and = 0. (b) When = /4 (45), the light is right elliptically polarized with a tilted major axis. (c) When = /2 (90), the light is right elliptically polarized. If Exo and Eyo were equal, this would be right circularly polarized light. © 1999 S.O ...
... (a) Linearly polarized light with Eyo = 2Exo and = 0. (b) When = /4 (45), the light is right elliptically polarized with a tilted major axis. (c) When = /2 (90), the light is right elliptically polarized. If Exo and Eyo were equal, this would be right circularly polarized light. © 1999 S.O ...
The Nature of Light
... determines what element a substance is. • Each element has a number of electrons equal to the number of protons • The electron orbitals are different for each element, and the energy differences between the orbitals are unique as well. • This means that if we can detect the energy emitted or absorbe ...
... determines what element a substance is. • Each element has a number of electrons equal to the number of protons • The electron orbitals are different for each element, and the energy differences between the orbitals are unique as well. • This means that if we can detect the energy emitted or absorbe ...
Optics01
... ~1590Zacharius Jensen (Netherlands). Constructed a compound microscope with a converging objective lens and a diverging eye lens 1604Johannes Kepler (Germany). In his book Ad Vitellionem Paralipomena, Kepler suggested that the intensity of light from a point source varies inversely with the square o ...
... ~1590Zacharius Jensen (Netherlands). Constructed a compound microscope with a converging objective lens and a diverging eye lens 1604Johannes Kepler (Germany). In his book Ad Vitellionem Paralipomena, Kepler suggested that the intensity of light from a point source varies inversely with the square o ...
slides introducing IR/Raman of proteins
... sufficient to ‘flip’ the spin of nuclei in a magnetic field (NMR). Nuclei interact weakly so spectral transitions between single, well defined energy levels are very sharp and well resolved. NMR is a vital technique for biological structure studies. • Higher energy microwaves can promote changes in ...
... sufficient to ‘flip’ the spin of nuclei in a magnetic field (NMR). Nuclei interact weakly so spectral transitions between single, well defined energy levels are very sharp and well resolved. NMR is a vital technique for biological structure studies. • Higher energy microwaves can promote changes in ...
Photoelectric Effect Practice Problems
... 1. Basic wave theory predicted that a blackbody should put out more energy at higher frequencies. In reality, above the peak frequency a real blackbody emits less radiation with increasing frequency. Planck was able to explain this contradiction by assuming that light energy is quantized and that hi ...
... 1. Basic wave theory predicted that a blackbody should put out more energy at higher frequencies. In reality, above the peak frequency a real blackbody emits less radiation with increasing frequency. Planck was able to explain this contradiction by assuming that light energy is quantized and that hi ...
Chemistry 4021/8021 Computational Chemistry 3/4 Credits Spring
... combination of CN π bonds with a weak antibonding interaction with Ni, and orbital 42 is a symmetric combination of CO π* antibonds with no significant Ni contribution (only a Ni pz orbital would have the right phase behavior to participate in this orbital, and no such orbital is nearby in energy). ...
... combination of CN π bonds with a weak antibonding interaction with Ni, and orbital 42 is a symmetric combination of CO π* antibonds with no significant Ni contribution (only a Ni pz orbital would have the right phase behavior to participate in this orbital, and no such orbital is nearby in energy). ...
gtse syllabus xii physics
... Current loop as a magnetic dipole and its magnetic dipole moment. Magnetic dipole moment of a revolving electron. Magnetic field intensity due to a magnetic dipole (bar magnet) along its axis and perpendicular to its axis. Torque on a magnetic dipole (bar magnet) in a uniform magnetic field; bar mag ...
... Current loop as a magnetic dipole and its magnetic dipole moment. Magnetic dipole moment of a revolving electron. Magnetic field intensity due to a magnetic dipole (bar magnet) along its axis and perpendicular to its axis. Torque on a magnetic dipole (bar magnet) in a uniform magnetic field; bar mag ...
Slide 1
... atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics, and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems, the B ...
... atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics, and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems, the B ...
You may recall the formula: V = W/q Potential difference between
... 3rd law doesn't agree with wave theroy KE should be determined by intensity rather than frequency (according to wave theory) ...
... 3rd law doesn't agree with wave theroy KE should be determined by intensity rather than frequency (according to wave theory) ...
Atomic Spectroscopy and the Bohr Model
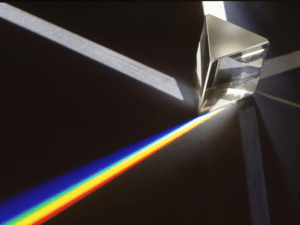
... emit the energy in the form of light energy (photons). • If we slow down this light using a prism or spectrometer, we can see the constituent colors that make up the color light that we are seeing. This series of lines is called the emission spectrum. This bright line spectrum is used to identify el ...
... emit the energy in the form of light energy (photons). • If we slow down this light using a prism or spectrometer, we can see the constituent colors that make up the color light that we are seeing. This series of lines is called the emission spectrum. This bright line spectrum is used to identify el ...
VNIR Reflectance Spectroscopy
... Why do we get spectra ? We can measure the light energy at the various wavelengths = a spectrum We examine the maxima and minima of spectral reflectance curves – minima are caused by molecular absorption, and we call these absorption features or absorption bands. Differences in absorption and scatt ...
... Why do we get spectra ? We can measure the light energy at the various wavelengths = a spectrum We examine the maxima and minima of spectral reflectance curves – minima are caused by molecular absorption, and we call these absorption features or absorption bands. Differences in absorption and scatt ...