Chapter 10: Simple Harmonic Motion
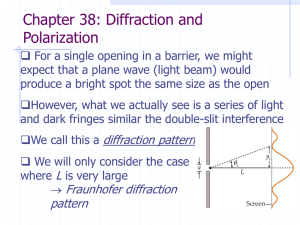
... illuminates a single slit 0.750 mm in width. (a) At what distance from the slit should a screen be located if the first minimum in the diffraction pattern is to be 0.850 mm from the center of the principal maximum? (b) What is the width of the central maximum? ...
... illuminates a single slit 0.750 mm in width. (a) At what distance from the slit should a screen be located if the first minimum in the diffraction pattern is to be 0.850 mm from the center of the principal maximum? (b) What is the width of the central maximum? ...
700 nm 400 nm Wavelength, λ Frequency, f 4x1014 Hz
... Because light rays do not actually pass through this image, it is known as a virtual image. In the case of a plane mirror, the image distance si from the mirror is the same as the object distance so from the mirror. The most common curved mirror is a spherical mirror. A concave or converging mirror ...
... Because light rays do not actually pass through this image, it is known as a virtual image. In the case of a plane mirror, the image distance si from the mirror is the same as the object distance so from the mirror. The most common curved mirror is a spherical mirror. A concave or converging mirror ...
Lectures 7-9 - U of L Class Index
... particles of matter can also behave as waves. Thus, his equation is not limited to electromagnetic radiation. In 1927, this was demonstrated by two separate experiments. Americans C.J. Davisson and L.H. Germer diffracted a beam of electrons through a nickel crystal, and Scot G.P. Thompson diffracted ...
... particles of matter can also behave as waves. Thus, his equation is not limited to electromagnetic radiation. In 1927, this was demonstrated by two separate experiments. Americans C.J. Davisson and L.H. Germer diffracted a beam of electrons through a nickel crystal, and Scot G.P. Thompson diffracted ...
Lectures 7-9
... particles of matter can also behave as waves. Thus, his equation is not limited to electromagnetic radiation. In 1927, this was demonstrated by two separate experiments. Americans C.J. Davisson and L.H. Germer diffracted a beam of electrons through a nickel crystal, and Scot G.P. Thompson diffracted ...
... particles of matter can also behave as waves. Thus, his equation is not limited to electromagnetic radiation. In 1927, this was demonstrated by two separate experiments. Americans C.J. Davisson and L.H. Germer diffracted a beam of electrons through a nickel crystal, and Scot G.P. Thompson diffracted ...
3 Radiation processes 3.1 Atomic and molecular structure
... Radiative transitions between discrete energy levels produce the line spectrum. The transition probabilities for dipole transitions are well described by the semiclassical formula (3.17). Because the life-time of the excited state is finite, the width of the line is also finite, ∆ν ∼ Γ, where Γ is t ...
... Radiative transitions between discrete energy levels produce the line spectrum. The transition probabilities for dipole transitions are well described by the semiclassical formula (3.17). Because the life-time of the excited state is finite, the width of the line is also finite, ∆ν ∼ Γ, where Γ is t ...
1H-NMR and 13C-NMR Spectra - Royal Society of Chemistry
... Optical Limiting Measurements The OL effect for the dimeric indium phthalocyanines 3, 4, and 5 has been studied with the Z-scan technique in the open aperture configuration. The samples are solutions of dimers in toluene with concentrations 0.8 - 110-4 M. The optical cell was a quartz cuvette ...
... Optical Limiting Measurements The OL effect for the dimeric indium phthalocyanines 3, 4, and 5 has been studied with the Z-scan technique in the open aperture configuration. The samples are solutions of dimers in toluene with concentrations 0.8 - 110-4 M. The optical cell was a quartz cuvette ...
Interference
... phase and have the same amplitude, they add destructively - the combined amplitude is zero. The result of adding two light wave amplitudes is called interference and can be observed in a variety of situations. ...
... phase and have the same amplitude, they add destructively - the combined amplitude is zero. The result of adding two light wave amplitudes is called interference and can be observed in a variety of situations. ...
Resonators and Mode Matching
... Determine the ABCD matrix for one round trip in the resonator matrix depends on starting point Solve equation ...
... Determine the ABCD matrix for one round trip in the resonator matrix depends on starting point Solve equation ...
Lectures 3-5
... •Electrons within an allowed orbital can move without radiating. •The orbital angular momentum of electrons in an atom is quantized (i.e. has a fixed set of allowed values). Only orbitals whose angular momentum is an integer multiple of h/2p are “allowed”. These orbitals are called stationary states ...
... •Electrons within an allowed orbital can move without radiating. •The orbital angular momentum of electrons in an atom is quantized (i.e. has a fixed set of allowed values). Only orbitals whose angular momentum is an integer multiple of h/2p are “allowed”. These orbitals are called stationary states ...
Argos TM - Lockheed Martin
... Figure 1: The QEPAS tuning-fork sensor. With QEPAS, researchers measure the amplitude of the sound wave that is generated when gas molecules absorb modulated light. The detection module is a low-cost, compact, quartz tuning fork, such as those commonly found in digital watches. The QEPAS sensor has ...
... Figure 1: The QEPAS tuning-fork sensor. With QEPAS, researchers measure the amplitude of the sound wave that is generated when gas molecules absorb modulated light. The detection module is a low-cost, compact, quartz tuning fork, such as those commonly found in digital watches. The QEPAS sensor has ...
Mechanisms of Radio Wave Emission
... rapidly. The ion attracts the electron but not strongly enough to capture it. As the charged particles are accelerated, they emit radio waves. ...
... rapidly. The ion attracts the electron but not strongly enough to capture it. As the charged particles are accelerated, they emit radio waves. ...
optical design of an echelle grating based atomic emission
... also lacks the flexibility of changing the analytical lines at will since the slits are positioned permanently. An echelle grating based spectrometer with a charge coupled device (CCD) detector overcomes these limitations by allowing continuous recording of lines in the entire wavelength band of the ...
... also lacks the flexibility of changing the analytical lines at will since the slits are positioned permanently. An echelle grating based spectrometer with a charge coupled device (CCD) detector overcomes these limitations by allowing continuous recording of lines in the entire wavelength band of the ...
germ free handle
... from the bottom of the sea to inside animals. fiber optics The use of thin, flexible fibers of glass (known as optical fibers) or other transparent solids to transmit light signals, chiefly for telecommunications. germ Any one-celled microorganism, such as a bacterium, fungal species or virus partic ...
... from the bottom of the sea to inside animals. fiber optics The use of thin, flexible fibers of glass (known as optical fibers) or other transparent solids to transmit light signals, chiefly for telecommunications. germ Any one-celled microorganism, such as a bacterium, fungal species or virus partic ...
Creative Molecules Inc. ADH
... Isotopic coding enables univocal detection of the crosslinked products in mass spectra. Reaction products of ADH-H8/D8 will manifest in mass spectra as doublets of peaks of equal intensity corresponding to light (H8) and heavy (D8) forms of the reagent separated by 8.05016 Da divided by charge state ...
... Isotopic coding enables univocal detection of the crosslinked products in mass spectra. Reaction products of ADH-H8/D8 will manifest in mass spectra as doublets of peaks of equal intensity corresponding to light (H8) and heavy (D8) forms of the reagent separated by 8.05016 Da divided by charge state ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.