Optical properties of silicon at low temperatures
... Institut für Festkörperphysik, Friedrich-Schiller-University Jena ...
... Institut für Festkörperphysik, Friedrich-Schiller-University Jena ...
Formulae, Equations Homework
... 3. Using the valency rules, write the chemical formula for the following compounds: a) Calcium chloride b) Potassium bromide c) Sodium sulphide d) Calcium oxide e) Aluminium oxide 4. Using valency rules and taking careful note of the roman numerals, write the chemical formula for the following compo ...
... 3. Using the valency rules, write the chemical formula for the following compounds: a) Calcium chloride b) Potassium bromide c) Sodium sulphide d) Calcium oxide e) Aluminium oxide 4. Using valency rules and taking careful note of the roman numerals, write the chemical formula for the following compo ...
Balmer_Prism2007
... description, specifically the derivation of chromatic resolving power, is for a perfectly sharp line source i.e. a very narrow slit. One uses the slits to vary the amount of light into the prism open the slit and more light. This is at a loss of effective chromatic resolving power but an increase in ...
... description, specifically the derivation of chromatic resolving power, is for a perfectly sharp line source i.e. a very narrow slit. One uses the slits to vary the amount of light into the prism open the slit and more light. This is at a loss of effective chromatic resolving power but an increase in ...
K. S. Al Mugren, Y. El Sayed, H. Shoukry, A. El Taher
... compositions; 60P2O5 –30ZnO- 10CuO in mol% (sample A), 55P2O5 – 30ZnO- 15CuO in mol% (sample B) and 50P2O5 – 30ZnO- 20CuO in mol% (sample C) have been melted in alumina crucibles, the temperature was probably between1000 and1100 °C. Composites were cast in a steel mold at room temperature followed b ...
... compositions; 60P2O5 –30ZnO- 10CuO in mol% (sample A), 55P2O5 – 30ZnO- 15CuO in mol% (sample B) and 50P2O5 – 30ZnO- 20CuO in mol% (sample C) have been melted in alumina crucibles, the temperature was probably between1000 and1100 °C. Composites were cast in a steel mold at room temperature followed b ...
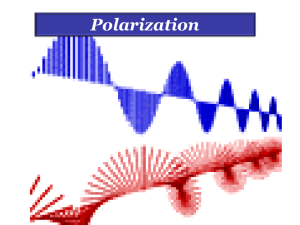
Polarization_1
... anisotropic crystal, it splits into two beams, each of them being characterized by a certain state of polarization. If by some method, we could eliminate one of the beams then we would obtained a LPL. ...
... anisotropic crystal, it splits into two beams, each of them being characterized by a certain state of polarization. If by some method, we could eliminate one of the beams then we would obtained a LPL. ...
EM Waves and Color
... It will slow down. Which type of medium will allow a sound wave to have the greatest KE? In a solid (an elastic solid) b/c the molecules are closer together. Sound requires a medium to transfer the energy. ...
... It will slow down. Which type of medium will allow a sound wave to have the greatest KE? In a solid (an elastic solid) b/c the molecules are closer together. Sound requires a medium to transfer the energy. ...
DG Papazoglou et al.
... D.G. Papazoglou et al., Opt. Lett. 31, 1441 (2006) , D. G. Papazoglou et al., Opt. Lett., 32, 2055 (2007), D.G. Papazoglou et al., (invited) to appear in Optical Materials Express (2011) ...
... D.G. Papazoglou et al., Opt. Lett. 31, 1441 (2006) , D. G. Papazoglou et al., Opt. Lett., 32, 2055 (2007), D.G. Papazoglou et al., (invited) to appear in Optical Materials Express (2011) ...
Equilibrium (PowerPoint) West Coast 2015
... Students will understand and appreciate the relationship between kinetics and equilibrium. ...
... Students will understand and appreciate the relationship between kinetics and equilibrium. ...
The Photoelectric Effect
... was first observed by Heinage) is applied between the plate and the rich Hertz in 1886, who found during cup such that the motion of the photoelectrons towards the cup is resisted (i.e. if one of his experiments that eleca single, motionless electron were placed in the area between the plate and the ...
... was first observed by Heinage) is applied between the plate and the rich Hertz in 1886, who found during cup such that the motion of the photoelectrons towards the cup is resisted (i.e. if one of his experiments that eleca single, motionless electron were placed in the area between the plate and the ...
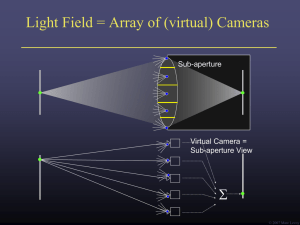
the measurement of the speed of the light
... face position respectively F1, F2-original and shifted focal points L- lens ...
... face position respectively F1, F2-original and shifted focal points L- lens ...
Tunable light emission from a boron nitride nanotube device
... Boron nitride is extremely efficient in ultraviolet light emission, one of the best materials currently available. However, boron nitride nanotubes emit light in a very limited range of the ultraviolet spectrum. This means they cannot be used in applications where the emission needs to be produced i ...
... Boron nitride is extremely efficient in ultraviolet light emission, one of the best materials currently available. However, boron nitride nanotubes emit light in a very limited range of the ultraviolet spectrum. This means they cannot be used in applications where the emission needs to be produced i ...
Essential Questions and Answers: What is light? Light is a form of
... Electromagnetic waves are produced by the motion of electrically charged particles. These waves are also called "electromagnetic radiation" because they radiate from the electrically charged particles. They travel through empty space as well as through air and other substances. ...
... Electromagnetic waves are produced by the motion of electrically charged particles. These waves are also called "electromagnetic radiation" because they radiate from the electrically charged particles. They travel through empty space as well as through air and other substances. ...
Physics 8.04 MIT September 19, 1373 Exercises
... 14. Slit width in an atomic beam apparatus In an atomic beam apparatus, potassium metal (atomic weight 39) is heated to its boiling point (T = 760° C ^ 1000° Kelvin) and streams out of the oven aperture as individual atoms (at average energy 53 kT ££ 1/10 eV, where k is Boltzmann f s constant = ...
... 14. Slit width in an atomic beam apparatus In an atomic beam apparatus, potassium metal (atomic weight 39) is heated to its boiling point (T = 760° C ^ 1000° Kelvin) and streams out of the oven aperture as individual atoms (at average energy 53 kT ££ 1/10 eV, where k is Boltzmann f s constant = ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.