Heat Transfer WebQuest Visit the Heat Transfer website (http://www
... Click on the picture of the pot. Light the burner and view the animation. What type of heat transfer is occurring? Define it. ...
... Click on the picture of the pot. Light the burner and view the animation. What type of heat transfer is occurring? Define it. ...
Heat stress round table
... implement a specific strategy during the carry over period? • What is, from your point of view, the main limiting factor to improve conception rate during the heat stress period? ...
... implement a specific strategy during the carry over period? • What is, from your point of view, the main limiting factor to improve conception rate during the heat stress period? ...
Modelling of wire die coating
... exchange between outer stream which could be waste dirty water and inner stream. The OPHE are often used for heat recovery purposes in laundries, paper industries etc. The main problem in thermo dynamical calculation of such type of heat exchanger is determination of the heat transfer coefficient on ...
... exchange between outer stream which could be waste dirty water and inner stream. The OPHE are often used for heat recovery purposes in laundries, paper industries etc. The main problem in thermo dynamical calculation of such type of heat exchanger is determination of the heat transfer coefficient on ...
Review of 17.1, 17.2 and 17.3 Name: 1.) When 2 moles of NO burn
... 3. In a study of the properties of chlorine, chlorine gas at 25 oC and standard pressure is cooled to a temperature of 150oC. (a) Sketch a cooling curve, including appropriate phase transition temperatures obtained from the periodic table. (b) Label each section of the graph in part (a) with one of ...
... 3. In a study of the properties of chlorine, chlorine gas at 25 oC and standard pressure is cooled to a temperature of 150oC. (a) Sketch a cooling curve, including appropriate phase transition temperatures obtained from the periodic table. (b) Label each section of the graph in part (a) with one of ...
Chapter 12 Laws of Thermodynamics
... • entropy is a quantitative description of the disorganization of a system. • 1 kg of water has more entropy than 1kg of ice because in the water the molecules are not organized into a regular crystal lattice. • 2nd Law of TD stated as entropy. The total entropy of a system in any physical process c ...
... • entropy is a quantitative description of the disorganization of a system. • 1 kg of water has more entropy than 1kg of ice because in the water the molecules are not organized into a regular crystal lattice. • 2nd Law of TD stated as entropy. The total entropy of a system in any physical process c ...
Conceptual Physics. Tenth Edition
... liquid or a gas. Convection is the result of changes in density in parts of a liquid or gas. This change in density is the result of change in temperature. This process is called natural convection whereas in a forced convection the speed of the heat transfer is being increased by wind or other forc ...
... liquid or a gas. Convection is the result of changes in density in parts of a liquid or gas. This change in density is the result of change in temperature. This process is called natural convection whereas in a forced convection the speed of the heat transfer is being increased by wind or other forc ...
Specific Heat of a Metal
... specific heat capacity of the metals that were found and also determine the identity of the metals (if possible). In this section state any sources of error and make reasonable suggestions as to how to improve the lab. ...
... specific heat capacity of the metals that were found and also determine the identity of the metals (if possible). In this section state any sources of error and make reasonable suggestions as to how to improve the lab. ...
Bagian 2 termodinamika
... and propels itself without the use of coal or oil in the following way. It pumps in warm sea water, extracts heat from that sea water, concentrates the extracted heat in its boilers, and discharges the cooled seawater back into the ocean. The discharged water may be ice if enough heat has been taken ...
... and propels itself without the use of coal or oil in the following way. It pumps in warm sea water, extracts heat from that sea water, concentrates the extracted heat in its boilers, and discharges the cooled seawater back into the ocean. The discharged water may be ice if enough heat has been taken ...
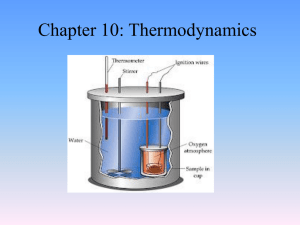
Chapter 10: Thermodynamics
... thermodynamic process that takes place at a constant volume so that no work is done on or by the system, ex: a car with closed windows parked in a hot garage. • Isothermal process: a thermodynamic process that takes place at a constant temperature, ex usually a slow process like a balloon expanding ...
... thermodynamic process that takes place at a constant volume so that no work is done on or by the system, ex: a car with closed windows parked in a hot garage. • Isothermal process: a thermodynamic process that takes place at a constant temperature, ex usually a slow process like a balloon expanding ...
Heat on the move
... Heat can move from one place to another – heat Put the three spoons into the container of hot is transferred. It always moves from somewhere water. Feel the ends of the spoons. Which of the or something hot to a place or object that’s cooler. ends became hot? Can you think of some examples? When hea ...
... Heat can move from one place to another – heat Put the three spoons into the container of hot is transferred. It always moves from somewhere water. Feel the ends of the spoons. Which of the or something hot to a place or object that’s cooler. ends became hot? Can you think of some examples? When hea ...
chapter 13 (Homework) - Tutor
... Calculate the heat required to vaporize 8.27 g of benzene at its normal boiling point Why is the heat of vaporization more than three times the heat of fusion? ...
... Calculate the heat required to vaporize 8.27 g of benzene at its normal boiling point Why is the heat of vaporization more than three times the heat of fusion? ...
Ch3_HeatTransfer_5
... • The conduction and convection heat transfer in engines are processes that occur in series and parallel with each other. A series path is convection through the cylinder gas boundary layer, conduction across the cylinder wall, and convection through the coolant liquid boundary layer; and a parallel ...
... • The conduction and convection heat transfer in engines are processes that occur in series and parallel with each other. A series path is convection through the cylinder gas boundary layer, conduction across the cylinder wall, and convection through the coolant liquid boundary layer; and a parallel ...
Currents experiment
... (lithosphere)…. What does tension look like? 2. Compression is s force the pushes on the plates (lithosphere). What does compression look like? 3. Shearing is a force that pushes on the plates causing one to move in one direction and the other plate to move in opposite direction. What does shearing ...
... (lithosphere)…. What does tension look like? 2. Compression is s force the pushes on the plates (lithosphere). What does compression look like? 3. Shearing is a force that pushes on the plates causing one to move in one direction and the other plate to move in opposite direction. What does shearing ...
Specific Heat Capacity - Cobequid Educational Centre
... or drinks in the cooler. Since heat leaves the food, it gets cold. 2. Preventing Frost Damage When a frost is predicted, farmers will turn on the water sprinklers. As the water falls on the plants and starts to freeze, heat is released to the surroundings and plants. The heat helps the plants stay ...
... or drinks in the cooler. Since heat leaves the food, it gets cold. 2. Preventing Frost Damage When a frost is predicted, farmers will turn on the water sprinklers. As the water falls on the plants and starts to freeze, heat is released to the surroundings and plants. The heat helps the plants stay ...
CHE 301 Problem set #3
... adding aqueous ammonia solution at a constant rate of 10 liters/min to an initial charge of formalin solution: 6HCHO + 4NH3 --> N4(CH2)6 + 6H2O The reaction is instantaneous, irreversible, and exothermic. The initial charge of formalin solution is 2000 liters with a formaldehyde (A) concentration, C ...
... adding aqueous ammonia solution at a constant rate of 10 liters/min to an initial charge of formalin solution: 6HCHO + 4NH3 --> N4(CH2)6 + 6H2O The reaction is instantaneous, irreversible, and exothermic. The initial charge of formalin solution is 2000 liters with a formaldehyde (A) concentration, C ...
Lesson
... will make initial and final temperature measurements of a hot metal object placed in a cup of water and determine the specific heat capacities of two different metals. Time Required: 50 minutes Group Size: 2-3 Cost to implement: The major cost comes from the digital thermometers and portable stove. ...
... will make initial and final temperature measurements of a hot metal object placed in a cup of water and determine the specific heat capacities of two different metals. Time Required: 50 minutes Group Size: 2-3 Cost to implement: The major cost comes from the digital thermometers and portable stove. ...
Chapter Two Atoms & The Periodic Table
... Bclosed system (heat only pass through) Cisolated system (no heat or mass transfer) ...
... Bclosed system (heat only pass through) Cisolated system (no heat or mass transfer) ...
Note Guide 7-4
... •Branch of chemistry that studies energy changes which take place during reactions and changes in state of matter = thermochemistry •Potential energy = energy of position/stored energy. But in chemistry we have chemical potential energy = energy stored in the chemical bonds of a substance --how much ...
... •Branch of chemistry that studies energy changes which take place during reactions and changes in state of matter = thermochemistry •Potential energy = energy of position/stored energy. But in chemistry we have chemical potential energy = energy stored in the chemical bonds of a substance --how much ...
Name: Date: ______ Thermochemistry Round Robin
... 2. How much heat energy is required to heat 20 g of platinum from 15˚C to 65˚C? The specific heat of platinum is 0.134 J/g˚C. ...
... 2. How much heat energy is required to heat 20 g of platinum from 15˚C to 65˚C? The specific heat of platinum is 0.134 J/g˚C. ...
Heat exchanger

A heat exchanger is a device used to transfer heat between one or more fluids. The fluids may be separated by a solid wall to prevent mixing or they may be in direct contact. They are widely used in space heating, refrigeration, air conditioning, power stations, chemical plants, petrochemical plants, petroleum refineries, natural-gas processing, and sewage treatment. The classic example of a heat exchanger is found in an internal combustion engine in which a circulating fluid known as engine coolant flows through radiator coils and air flows past the coils, which cools the coolant and heats the incoming air.