Thermodynamics - StrikerPhysics
... • Thermo (heat) dynamics (transfer) • Thermodynamic systems describe many many particles (molecules) which obey Newton’s laws for dynamics but which would be difficult to analyze due to their numbers. • We use macroscopic means for analysis of these systems of many particles - involving quantities s ...
... • Thermo (heat) dynamics (transfer) • Thermodynamic systems describe many many particles (molecules) which obey Newton’s laws for dynamics but which would be difficult to analyze due to their numbers. • We use macroscopic means for analysis of these systems of many particles - involving quantities s ...
Heat Transfer: Conduction, Convection and Latent Heat In addition
... warmed by contact with the ground and moistened by evaporation ...
... warmed by contact with the ground and moistened by evaporation ...
AS90184 - NBCPhyyear11
... A thermometer is usually constructed of glass with a bulb at one end containing a reservoir of coloured liquid. There is a thin capillary running the length of the thermometer. Heat applied to the bulb transfers easily through the thin outside shell of the bulb and heats the liquid within. As heat e ...
... A thermometer is usually constructed of glass with a bulb at one end containing a reservoir of coloured liquid. There is a thin capillary running the length of the thermometer. Heat applied to the bulb transfers easily through the thin outside shell of the bulb and heats the liquid within. As heat e ...
First Law of Thermodynamics
... Changes in internal energy are then associated with any energy that flows into or out of the system. We discussed two forms of energy flow: heat and temperature Heat. Heat is not temperature. Can't say this enough. Heat is not temperature. Heat is energy flow. We will show with the second law that h ...
... Changes in internal energy are then associated with any energy that flows into or out of the system. We discussed two forms of energy flow: heat and temperature Heat. Heat is not temperature. Can't say this enough. Heat is not temperature. Heat is energy flow. We will show with the second law that h ...
heat engine
... In general, irreversible processes cause us to lose some, but not necessarily all, of the ability to do work. This partial loss can be expressed in terms of a concept called entropy. ...
... In general, irreversible processes cause us to lose some, but not necessarily all, of the ability to do work. This partial loss can be expressed in terms of a concept called entropy. ...
4.1 The Concepts of Force and Mass
... In general, irreversible processes cause us to lose some, but not necessarily all, of the ability to do work. This partial loss can be expressed in terms of a concept called entropy. ...
... In general, irreversible processes cause us to lose some, but not necessarily all, of the ability to do work. This partial loss can be expressed in terms of a concept called entropy. ...
Thermodynamics
... A device that changes heat energy to useful work is called a heat engine. A steam engine and an internal combustion engine are both examples of a heat engine You do not need to know the details of how these work. We just draw a very simple diagram. Efficiency = ΔW/Qhot ...
... A device that changes heat energy to useful work is called a heat engine. A steam engine and an internal combustion engine are both examples of a heat engine You do not need to know the details of how these work. We just draw a very simple diagram. Efficiency = ΔW/Qhot ...
BUOYANCY-DRIVEN TURBULENT CONVECTION IN A BUNDLE
... heated cylinders, where each cylinder releases an equal, uniform heat flux distribution q 00 and a Boussinesq approximation is introduced to represent buoyancy effects. Periodic boundary conditions are enforced along the cross flow directions y and z as well as the streamwise direction x on all comp ...
... heated cylinders, where each cylinder releases an equal, uniform heat flux distribution q 00 and a Boussinesq approximation is introduced to represent buoyancy effects. Periodic boundary conditions are enforced along the cross flow directions y and z as well as the streamwise direction x on all comp ...
Heat
... 1. A 15.75-g piece of iron absorbs 1086.75 J of heat energy, and its temperature changes from 25°C to 175°C. Calculate the heat capacity of iron. ...
... 1. A 15.75-g piece of iron absorbs 1086.75 J of heat energy, and its temperature changes from 25°C to 175°C. Calculate the heat capacity of iron. ...
Heat Exhaustion
... Risk Factors for Heat Exhaustion Heat exhaustion is strongly related to the heat index, which is a measurement of how hot you feel when the effects of relative humidity and air temperature are combined. A relative humidity of 60% or more hampers sweat evaporation, which hinders your body's ability t ...
... Risk Factors for Heat Exhaustion Heat exhaustion is strongly related to the heat index, which is a measurement of how hot you feel when the effects of relative humidity and air temperature are combined. A relative humidity of 60% or more hampers sweat evaporation, which hinders your body's ability t ...
Reversible and irreversible Processes
... Reversible process: can be defined as one whose “direction” can be reversed by an infinitesimal small change in some property of the system. “Gedankenexperiment” to picture a reversible process: ...
... Reversible process: can be defined as one whose “direction” can be reversed by an infinitesimal small change in some property of the system. “Gedankenexperiment” to picture a reversible process: ...
Nats 101 S00 #8
... Wood feels warm, or normal, while metal usually feels cold. Actually both are at the same temperature, its just that the metal conducts the heat better, thereby taking heat from your hand, making it feel cooler. 2. Convection: This is the process of moving heat around by moving the molecules that co ...
... Wood feels warm, or normal, while metal usually feels cold. Actually both are at the same temperature, its just that the metal conducts the heat better, thereby taking heat from your hand, making it feel cooler. 2. Convection: This is the process of moving heat around by moving the molecules that co ...
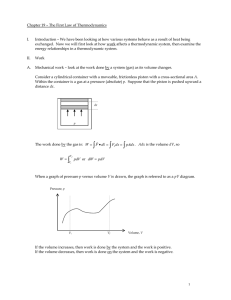
Chapter 19 – The First Law of Thermodynamics
... Interesting Note: If a system changes from an initial state i to a final state f along different paths (e.g., Path A and Path B), the change in internal energy will be the same along those paths. And, in fact, all paths that go from i to f. That is, U = Uf - Ui . From the first law, that means that ...
... Interesting Note: If a system changes from an initial state i to a final state f along different paths (e.g., Path A and Path B), the change in internal energy will be the same along those paths. And, in fact, all paths that go from i to f. That is, U = Uf - Ui . From the first law, that means that ...
Treatment of Heat Illness
... ff Using as many people as possible, lift the patient into the tub ff Some patients may become aggressive and will need to be restrained in the tub ff Immerse as much of the body as possible ff Support the patient in the tub by looping a towel under his/her arms ff Cover the patient’s head with a we ...
... ff Using as many people as possible, lift the patient into the tub ff Some patients may become aggressive and will need to be restrained in the tub ff Immerse as much of the body as possible ff Support the patient in the tub by looping a towel under his/her arms ff Cover the patient’s head with a we ...
Heat Transfer/ Specific Heat Problems Worksheet
... Heat Transfer/ Specific Heat Problems Worksheet Solving For Heat (q) 1. How many joules of heat are required to raise the temperature of 550 g of water from 12.0 oC to 18.0 oC? 2. How much heat is lost when a 64 g piece of copper cools from 375 oC, to 26 oC? (The specific heat of copper is 0.38452 J ...
... Heat Transfer/ Specific Heat Problems Worksheet Solving For Heat (q) 1. How many joules of heat are required to raise the temperature of 550 g of water from 12.0 oC to 18.0 oC? 2. How much heat is lost when a 64 g piece of copper cools from 375 oC, to 26 oC? (The specific heat of copper is 0.38452 J ...
Ch 10 Review activity
... 4. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67.4 kJ of heat, and its temperature changes from 32°C to 57°C. When this piece of wood is burned, would the combustion reaction be endothermic or exothermic? Where does the energy that would be transferred in this re ...
... 4. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67.4 kJ of heat, and its temperature changes from 32°C to 57°C. When this piece of wood is burned, would the combustion reaction be endothermic or exothermic? Where does the energy that would be transferred in this re ...
Phase Changes
... their particle size. Therefore, gases volume is negligible. And gases are easily compressed. 0 Gases do not have attractive or repulsive forces between molecules. 0 Collisions between molecules can transfer energy but the total energy of the system is constant. This is called an ...
... their particle size. Therefore, gases volume is negligible. And gases are easily compressed. 0 Gases do not have attractive or repulsive forces between molecules. 0 Collisions between molecules can transfer energy but the total energy of the system is constant. This is called an ...
Chap19Class2
... 19-5 Latent Heat Example 19-6: Determining a latent heat. The specific heat of liquid mercury is 140 J/kg·°C. When 1.0 kg of solid mercury at its melting point of -39°C is placed in a 0.50-kg aluminum calorimeter filled with 1.2 kg of water at 20.0°C, the mercury melts and the final temperature of ...
... 19-5 Latent Heat Example 19-6: Determining a latent heat. The specific heat of liquid mercury is 140 J/kg·°C. When 1.0 kg of solid mercury at its melting point of -39°C is placed in a 0.50-kg aluminum calorimeter filled with 1.2 kg of water at 20.0°C, the mercury melts and the final temperature of ...
Thermodynamics - Bowles Physics
... Carnot Efficiency Carnot a believed that there was an absolute zero of temperature, from which he figured out that on being cooled to absolute zero, the fluid would give up all its heat energy. Therefore, if it falls only half way to absolute zero from its beginning temperature, it will give up hal ...
... Carnot Efficiency Carnot a believed that there was an absolute zero of temperature, from which he figured out that on being cooled to absolute zero, the fluid would give up all its heat energy. Therefore, if it falls only half way to absolute zero from its beginning temperature, it will give up hal ...
AP Physics B Thermodynamics
... Carnot Efficiency Carnot a believed that there was an absolute zero of temperature, from which he figured out that on being cooled to absolute zero, the fluid would give up all its heat energy. Therefore, if it falls only half way to absolute zero from its beginning temperature, it will give up hal ...
... Carnot Efficiency Carnot a believed that there was an absolute zero of temperature, from which he figured out that on being cooled to absolute zero, the fluid would give up all its heat energy. Therefore, if it falls only half way to absolute zero from its beginning temperature, it will give up hal ...
15.3 The First Law of Thermodynamics
... which the pressure, volume, and temperature of a gas are changed along the straight line in the figure. ...
... which the pressure, volume, and temperature of a gas are changed along the straight line in the figure. ...
1 - Southwest High School
... 2.) A 5 g sample of metal with a specific heat of 350 c / g oC is heated and the temperature changes by 10 oC. How much heat does the material gain? In this question, what is the unknown variable? __________ In this question, what is the value for m ? __________ In this question, what is the value f ...
... 2.) A 5 g sample of metal with a specific heat of 350 c / g oC is heated and the temperature changes by 10 oC. How much heat does the material gain? In this question, what is the unknown variable? __________ In this question, what is the value for m ? __________ In this question, what is the value f ...
Heat exchanger

A heat exchanger is a device used to transfer heat between one or more fluids. The fluids may be separated by a solid wall to prevent mixing or they may be in direct contact. They are widely used in space heating, refrigeration, air conditioning, power stations, chemical plants, petrochemical plants, petroleum refineries, natural-gas processing, and sewage treatment. The classic example of a heat exchanger is found in an internal combustion engine in which a circulating fluid known as engine coolant flows through radiator coils and air flows past the coils, which cools the coolant and heats the incoming air.