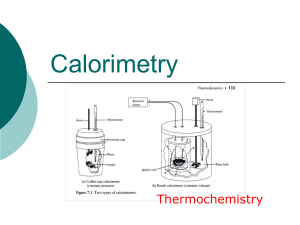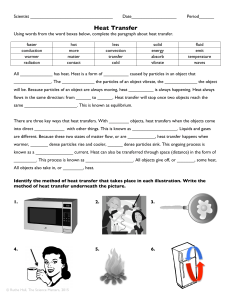
Heat Transfer - Granville County Public Schools
... same _____________________. This is known as equilibrium. There are three key ways that heat transfers. With ________ objects, heat transfers when the objects come into direct ____________ with other things. This is known as __________________. Liquids and gases are different. Because these two stat ...
... same _____________________. This is known as equilibrium. There are three key ways that heat transfers. With ________ objects, heat transfers when the objects come into direct ____________ with other things. This is known as __________________. Liquids and gases are different. Because these two stat ...
LECTURE 5 Temperature Scales The equation of state of any
... This is valid irrespective of the details of the interactions which bring about the ferromagnetic behavior and irrespective of the temperature dependence of C(T ). Extensive and Intensive Parameters The macroscopic parameters specifying the macrostate of a homogeneous system can be classified into t ...
... This is valid irrespective of the details of the interactions which bring about the ferromagnetic behavior and irrespective of the temperature dependence of C(T ). Extensive and Intensive Parameters The macroscopic parameters specifying the macrostate of a homogeneous system can be classified into t ...
Analysis of Heat Transfer in Rectangular
... θ = Temperature difference, [k] Subscripts conv = convection ch = channel sp = single phase bot = bottom ƒ = fluid i = inlet o =outlet Micro-channel Heat transfer has the very potential of wide applications in cooling high power density microchips in the CPU system, the micro power systems and even ...
... θ = Temperature difference, [k] Subscripts conv = convection ch = channel sp = single phase bot = bottom ƒ = fluid i = inlet o =outlet Micro-channel Heat transfer has the very potential of wide applications in cooling high power density microchips in the CPU system, the micro power systems and even ...
Heat - Denton ISD
... The total heat involved during a phase change depends on the latent heat, L and on the total mass of the substance: Q = mL. (Q is the heat required or given off during the phase change. ...
... The total heat involved during a phase change depends on the latent heat, L and on the total mass of the substance: Q = mL. (Q is the heat required or given off during the phase change. ...
An Approach to a Zero
... Another approach is to use the walls or windows as heat exchangers, an attractive example of integration if it can be made to work. Walls naturally work in this way, to some extent: air that leaks in through a wall picks up some heat that would otherwise flow out by conduction. Facades in high-tech ...
... Another approach is to use the walls or windows as heat exchangers, an attractive example of integration if it can be made to work. Walls naturally work in this way, to some extent: air that leaks in through a wall picks up some heat that would otherwise flow out by conduction. Facades in high-tech ...
Lecture 32 - PhysicsGivesYouWings
... • Summary of results from kinetic theory: – Pressure: – Temperature: – Internal energy: – Molar isochoric specific heat capacity: – Molar isobaric specific heat capacity: ...
... • Summary of results from kinetic theory: – Pressure: – Temperature: – Internal energy: – Molar isochoric specific heat capacity: – Molar isobaric specific heat capacity: ...
Section 2.3 Day 2
... Remember, specific heat is the amount of energy that must be transferred as heat to raise the temperature of 1 g of a substance by 1K or 1°C. The amount of energy depends on: The nature of the material The mass of the material The size of the temperature change ...
... Remember, specific heat is the amount of energy that must be transferred as heat to raise the temperature of 1 g of a substance by 1K or 1°C. The amount of energy depends on: The nature of the material The mass of the material The size of the temperature change ...
Energy Worksheet - MICDS Intranet Menu
... A 5.0 kilogram block of ice at -10.0oC is placed in a container of warm water. The entire block of ice is warmed to 0.0oC, and 4.0 kilograms of ice remains unmelted. At this point, how many joules were transferred from the warm water completely? q = mct = 5000gr x 2.09 J/gr oC x (-10oC - 0oC) = - 10 ...
... A 5.0 kilogram block of ice at -10.0oC is placed in a container of warm water. The entire block of ice is warmed to 0.0oC, and 4.0 kilograms of ice remains unmelted. At this point, how many joules were transferred from the warm water completely? q = mct = 5000gr x 2.09 J/gr oC x (-10oC - 0oC) = - 10 ...
Thermal Chem Review and Key
... 2. Compare the potential chemical energy of reactants and products for both endo and exothermic reactions. (Look at your notes with the chemical potential energy diagrams for endo & exothermic reactions.) 3. Define enthalpy and explain the sign (+ or -) for endo and exothermic reactions. 4. Define c ...
... 2. Compare the potential chemical energy of reactants and products for both endo and exothermic reactions. (Look at your notes with the chemical potential energy diagrams for endo & exothermic reactions.) 3. Define enthalpy and explain the sign (+ or -) for endo and exothermic reactions. 4. Define c ...
Chapter 6 Thermal Energy
... In the simplest and oldest heating system, wood or coal is burned in a stove The heat that is produced by the burning fuel is transferred from the stove to the surrounding air by conduction, convection, and radiation. One disadvantage of this system is that heat transfer from the room in which ...
... In the simplest and oldest heating system, wood or coal is burned in a stove The heat that is produced by the burning fuel is transferred from the stove to the surrounding air by conduction, convection, and radiation. One disadvantage of this system is that heat transfer from the room in which ...
- Uponorpro.com
... the strategies used in forcedair systems are not necessarily applicable for radiant systems. The way in which energy is evaluated and managed is on a more finite level with radiant systems. The temperature in one room will not impact the temperature in the next room. This is why it is easier and les ...
... the strategies used in forcedair systems are not necessarily applicable for radiant systems. The way in which energy is evaluated and managed is on a more finite level with radiant systems. The temperature in one room will not impact the temperature in the next room. This is why it is easier and les ...
GeoT*SOL® Exploiting the Earth`s Sustainable Energy Supply
... It’s your choice: heat sources and system types GeoT*SOL® basic supports the following heat pump configurations: Brine/Water – Brine/water heat pumps with geothermal probes extract heat stored in lower ground layers. This requires one or more holes drilled vertically into the ground. Near-surface h ...
... It’s your choice: heat sources and system types GeoT*SOL® basic supports the following heat pump configurations: Brine/Water – Brine/water heat pumps with geothermal probes extract heat stored in lower ground layers. This requires one or more holes drilled vertically into the ground. Near-surface h ...
Page 1 of 2 Gerbing`s Heated Clothing // How it Works 02/11/2009
... Being as tiny as they are, and made from stainless steel, our strands (and therefore our wire) is incredibly flexible and exceptionally rugged. Also, at about .025” (vs. about .075 for the copper resistance wire that’s been in use all these years), Microwire is exceptionally thin. So much so you can ...
... Being as tiny as they are, and made from stainless steel, our strands (and therefore our wire) is incredibly flexible and exceptionally rugged. Also, at about .025” (vs. about .075 for the copper resistance wire that’s been in use all these years), Microwire is exceptionally thin. So much so you can ...
Alfa Laval wins important order for mineral processing NEWS
... The Stanwell magnesium plant will produce 90,000 tons primary magnesium metal per annum. Predominantly the users of this product will be the automotive industry. One of the processes will be used to make anhydrous magnesium chloride as the feed to the electrolytic cells. The 84 spiral heat exchanger ...
... The Stanwell magnesium plant will produce 90,000 tons primary magnesium metal per annum. Predominantly the users of this product will be the automotive industry. One of the processes will be used to make anhydrous magnesium chloride as the feed to the electrolytic cells. The 84 spiral heat exchanger ...
SPECIFIC HEAT CAPACITY
... 7. What is the specific heat of an unknown substance if a 2.50 g sample releases 12 cal as its temperature changes from 25°C to 20°C? 8. A piece of metal with a mass of 4.68 g absorbs 256 J of heat when its temperature increases by 182°C. What is the specific heat of the metal? 9. If 335 g water at ...
... 7. What is the specific heat of an unknown substance if a 2.50 g sample releases 12 cal as its temperature changes from 25°C to 20°C? 8. A piece of metal with a mass of 4.68 g absorbs 256 J of heat when its temperature increases by 182°C. What is the specific heat of the metal? 9. If 335 g water at ...
U3 S1 L2 q=mct
... 1. Calculate the heat change involved when 2.00 L of water is heated from 20.0°C to 99.7°C in an electric kettle. 2. Calculate the heat change associated with cooling a 350.0 g aluminum bar from 70.0°C to 25.0°C. Is the change endothermic or exothermic? Why? (Hint: what is the sign of your answer?) ...
... 1. Calculate the heat change involved when 2.00 L of water is heated from 20.0°C to 99.7°C in an electric kettle. 2. Calculate the heat change associated with cooling a 350.0 g aluminum bar from 70.0°C to 25.0°C. Is the change endothermic or exothermic? Why? (Hint: what is the sign of your answer?) ...
AP Ch 06 apchapt6r
... 6.1 Nature of Energy • The ability to do work or produce heat. • Conserved - can be converted from one form to another but can neither be created nor destroyed. • Work is a force acting over a distance. • Potential: due to position or composition - can be converted to work. • Kinetic: due to motion ...
... 6.1 Nature of Energy • The ability to do work or produce heat. • Conserved - can be converted from one form to another but can neither be created nor destroyed. • Work is a force acting over a distance. • Potential: due to position or composition - can be converted to work. • Kinetic: due to motion ...
Progress Report - UCLA Fusion Home
... • Generating and transporting heat energy • Shielding nuclear radiation • Producing and recovering Tritium Fig. 1. 3D view of FFHR ...
... • Generating and transporting heat energy • Shielding nuclear radiation • Producing and recovering Tritium Fig. 1. 3D view of FFHR ...
Heat exchanger

A heat exchanger is a device used to transfer heat between one or more fluids. The fluids may be separated by a solid wall to prevent mixing or they may be in direct contact. They are widely used in space heating, refrigeration, air conditioning, power stations, chemical plants, petrochemical plants, petroleum refineries, natural-gas processing, and sewage treatment. The classic example of a heat exchanger is found in an internal combustion engine in which a circulating fluid known as engine coolant flows through radiator coils and air flows past the coils, which cools the coolant and heats the incoming air.