* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Thermochemistry - hrsbstaff.ednet.ns.ca
Survey
Document related concepts
Water heating wikipedia , lookup
Thermoregulation wikipedia , lookup
Dynamic insulation wikipedia , lookup
Heat exchanger wikipedia , lookup
Intercooler wikipedia , lookup
Building insulation materials wikipedia , lookup
Solar air conditioning wikipedia , lookup
Heat equation wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Cogeneration wikipedia , lookup
Solar water heating wikipedia , lookup
R-value (insulation) wikipedia , lookup
Transcript
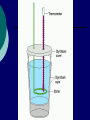
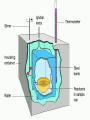
Calorimetry Thermochemistry Calorimeter Instrument used to measure amount of energy involved in a chemical reaction. It is equivalent to an isolated or closed system. (nothing may enter or exit the system) The energy change is not measured within the system, but the energy transferred to its surroundings. A basic calorimeter Two Styrofoam cups nestled within one another (insulation), then filled with a specific quantity of water and covered with another cup as a cover. A chemical reaction or phase change takes place inside and a thermometer is placed within to measure any change in temperature that occurs to the system. Assumptions 1. 2. 3. It is an isolated or closed system and there is no heat transfer between the calorimeter and its surroundings. The amount of heat absorbed or released by the calorimeter itself is too small to influence calculations. Any dilute solutions involved in the reaction are treated as if they are water. Bomb calorimeter Cannot use basic design for combustion reactions. Used in research for H for fuels, oils, food, explosives… Larger and more sophisticated The reaction container is strong enough to with stand an explosion, hence the name “bomb” Have fixed components, like volume of water, thermometer… Heavily insulated or vacuum insulated so no convection or conduction can occur affecting the enthalpy of the system. Often use the equation: q=CΔT Ccalorimetre: Ctotal = Cwater + Cthermometre +Cstirrer + Ccontainer A Bomb Calorimeter The main idea: qsystem =-qsurroundings System = reaction Surroundings = calorimeter (water) Solving calorimeter questions: For a regular calorimeter (not bomb): Heat released by the reaction = heat absorbed by the calorimeter + heat absorbed by water in the calorimeter q = mcΔT(calorimeter) + mcΔT(water) Note: When a styrene (Styrofoam) cup is used, often it is ignored in the calculations. It is assumed that the heat absorbed by the cup is negligible. If the heat absorbed by the cup should be included in the calculations, information about the heat capacity or specific heat capacity will be given in the question. Example: A 92.0g sample of a substance with a temperature of 55ºC is placed in a large scale polystyrene calorimeter. It contains 1.00 kg of water at 20.0ºC. The final temperature of the system is 25.2ºC. a) How much heat did the substance release? b) What is the specific heat capacity of the substance? You can assume the amount of energy absorbed by the polystyrene is negligible. So…calculate heat gained by water q = mcΔT = (1000g)(4.18)(25.2-20) = 21736 J (absorbed by water) a) Therefore substance lost (-) 21736J b) q = mcΔT -21736 = (92)c(25.2-55) -21736 = c -2741 7.9 J/g ·ºC = c Problems Pg 664 #1 4 WS: Calorimetry