t-75 mid-range heat transfer compound
... for its smooth texture and exceptional bonding strength. The smooth texture allows for easy spreading over surface heaters such as Thermon HeetSheet® tank heating units and other plate type coils for applications above the temperature limits of Non-Hardening compounds-375°F (190°C). The compound may ...
... for its smooth texture and exceptional bonding strength. The smooth texture allows for easy spreading over surface heaters such as Thermon HeetSheet® tank heating units and other plate type coils for applications above the temperature limits of Non-Hardening compounds-375°F (190°C). The compound may ...
1 CHAPTER 1 INTRODUCTORY REMARKS 1.1 Introduction
... The “calories” that nutritionists quote when talking about the calorific value of foods, is actually the kilocalorie and it is sometimes (but by no means always) written Calorie, with a capital C. How much simpler it would all be if all of us just used joules! There is yet another problem associated ...
... The “calories” that nutritionists quote when talking about the calorific value of foods, is actually the kilocalorie and it is sometimes (but by no means always) written Calorie, with a capital C. How much simpler it would all be if all of us just used joules! There is yet another problem associated ...
First Law of Thermodynamics
... Irreversible process is one in which thermal system’s changes cannot be retraced, such as gas expanding to fill a vacuum through an open stopcock. A thermodynamic system can transfer its internal energy by changing the temperature (or phase) of another system of it can use its internal energy to do ...
... Irreversible process is one in which thermal system’s changes cannot be retraced, such as gas expanding to fill a vacuum through an open stopcock. A thermodynamic system can transfer its internal energy by changing the temperature (or phase) of another system of it can use its internal energy to do ...
Introduction - HCC Learning Web
... joules/ (kg∙K) . Water has a relatively high specific heat of 1cal/(gm∙C). Metals usually have a low specific heat, for example lead has a specific heat of 0.03 cal/(gm∙C). A calorimeter is an instrument for determining the amount of heat evolved, transferred, or absorbed. In our case it will cons ...
... joules/ (kg∙K) . Water has a relatively high specific heat of 1cal/(gm∙C). Metals usually have a low specific heat, for example lead has a specific heat of 0.03 cal/(gm∙C). A calorimeter is an instrument for determining the amount of heat evolved, transferred, or absorbed. In our case it will cons ...
Influence of the ambient temperature during heat pipe
... of human error and minimize the impact of inertia radiator. Taking simply the heat pipes and the use thereof, it is obvious that the tube filled with water, even if they have better transport properties such as ethanol, are not suitable for application in the external environment in our geographic a ...
... of human error and minimize the impact of inertia radiator. Taking simply the heat pipes and the use thereof, it is obvious that the tube filled with water, even if they have better transport properties such as ethanol, are not suitable for application in the external environment in our geographic a ...
heat
... BD is an isobaric system (P is constant) P-V diagrams that are closed represent cyclical thermodynamic systems. Examples ...
... BD is an isobaric system (P is constant) P-V diagrams that are closed represent cyclical thermodynamic systems. Examples ...
heat
... BD is an isobaric system (P is constant) P-V diagrams that are closed represent cyclical thermodynamic systems. Examples ...
... BD is an isobaric system (P is constant) P-V diagrams that are closed represent cyclical thermodynamic systems. Examples ...
Mr Alasdair Ross at Southpointe Academy: Math and Chemistry Pages
... The standard state of a gas is the pure gas behaving as an ideal gas at 1 atm pressure and the temperature of interest (usually 25ºC). The standard enthalpy of reaction ( H ) is the enthalpy change for a reaction in which the reactants in their standard states yield products in their standard sta ...
... The standard state of a gas is the pure gas behaving as an ideal gas at 1 atm pressure and the temperature of interest (usually 25ºC). The standard enthalpy of reaction ( H ) is the enthalpy change for a reaction in which the reactants in their standard states yield products in their standard sta ...
L14
... combined system. For our simple system of two boxes, it simply requires that heat flows from the hotter to the colder system. But when we include work, phase changes, and chemical reactions, the bookkeeping gets a bit more complicated. Statistical mechanics arrives at the same relationship for entr ...
... combined system. For our simple system of two boxes, it simply requires that heat flows from the hotter to the colder system. But when we include work, phase changes, and chemical reactions, the bookkeeping gets a bit more complicated. Statistical mechanics arrives at the same relationship for entr ...
Thermodynamics
... In thermodynamics, “system” still means that. However, we add the notion that the system will usually include some definite amount of a fluid – typically, an ideal gas. It might also include other elements, such as the fluid’s container. It’s always important to be clear about what’s in the system, ...
... In thermodynamics, “system” still means that. However, we add the notion that the system will usually include some definite amount of a fluid – typically, an ideal gas. It might also include other elements, such as the fluid’s container. It’s always important to be clear about what’s in the system, ...
Liquids
... It is usually assumed that more heat means higher temperature, but not when changing phase. EX:1 If a sample of water at 20.0 oC is heated by a hot plate that gives off 250.0 J, how grams of water are in the sample if the temperature rises to 30.0 oC? ...
... It is usually assumed that more heat means higher temperature, but not when changing phase. EX:1 If a sample of water at 20.0 oC is heated by a hot plate that gives off 250.0 J, how grams of water are in the sample if the temperature rises to 30.0 oC? ...
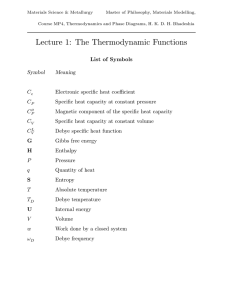
Thermodynamic functions - Phase Transformations Group
... is found to vary slightly with temperature. In spite of this, the Debye function frequently can be used quite accurately for CVL {T } if an average TD is calculated for the range TD /6 − TD . ...
... is found to vary slightly with temperature. In spite of this, the Debye function frequently can be used quite accurately for CVL {T } if an average TD is calculated for the range TD /6 − TD . ...
lecture21
... absorbs energy Q1 as heat from a thermal reservoir at TH. Equivalent amount of work W(W=Q1) is performed. Consider another device 2 operating as a cycle, which absorbs energy QL as heat from a low temperature thermal reservoir at TL and rejects energy QH (QH=QL+W). Such a device does not violate Cla ...
... absorbs energy Q1 as heat from a thermal reservoir at TH. Equivalent amount of work W(W=Q1) is performed. Consider another device 2 operating as a cycle, which absorbs energy QL as heat from a low temperature thermal reservoir at TL and rejects energy QH (QH=QL+W). Such a device does not violate Cla ...
Lecture 36.Thermodyn..
... The baseball field, with the lower specific heat, will change temperature more readily, so it will cool off faster. The high specific heat of concrete allows it to “retain heat” better and so it will not cool off so quickly—it has a higher “thermal inertia.” ...
... The baseball field, with the lower specific heat, will change temperature more readily, so it will cool off faster. The high specific heat of concrete allows it to “retain heat” better and so it will not cool off so quickly—it has a higher “thermal inertia.” ...
Chapter 6 Exam Study Guide Word document
... Honors Chemistry Chapter 6 – Thermochemistry Exam Study Guide Big Idea: Energy is exchanged or transformed in all chemical reactions and physical changes of matter. Learning Objectives: Students should be able to: ...
... Honors Chemistry Chapter 6 – Thermochemistry Exam Study Guide Big Idea: Energy is exchanged or transformed in all chemical reactions and physical changes of matter. Learning Objectives: Students should be able to: ...
Convection Currents and the Mantle
... stratosphere (tough liquid part of the outer mantle) and the lithosphere (the stiffer outer mantle and the crust). Because of the intense pressure and temperature in the mantle convection currents occur. To learn about what influence these convection currents have on Earth, read the Web page below a ...
... stratosphere (tough liquid part of the outer mantle) and the lithosphere (the stiffer outer mantle and the crust). Because of the intense pressure and temperature in the mantle convection currents occur. To learn about what influence these convection currents have on Earth, read the Web page below a ...
Document
... Imagine two systems A and B, separated by an adiabatic wall, while each is in contact with a third system C, via a conducting wall ]. The states of the systems change until both A and B come to thermal equilibrium with C. After this has happened if the adiabatic wall between A and B is replaced by a ...
... Imagine two systems A and B, separated by an adiabatic wall, while each is in contact with a third system C, via a conducting wall ]. The states of the systems change until both A and B come to thermal equilibrium with C. After this has happened if the adiabatic wall between A and B is replaced by a ...
Heat Transfer - Concord Consortium
... and the area of a surface such as a wall. If a house had ten times as much wall area as it had window area, and the wall was ten times as insulating, what would be the relative heat loss from wall and window? They would be the same, because the higher conductivity of one balances the greater area of ...
... and the area of a surface such as a wall. If a house had ten times as much wall area as it had window area, and the wall was ten times as insulating, what would be the relative heat loss from wall and window? They would be the same, because the higher conductivity of one balances the greater area of ...
appendix d - Florida Building Commission
... components, as defined in Section 3.1 for residential potable water heating. 2.2 Exclusion. This standard does not apply to desupereater/water heaters supplied as components of factory assembled refrigeration or air conditioning units. SECTION 3 DEFINITIONS 3.1 Desuperheater/water heater. A factory- ...
... components, as defined in Section 3.1 for residential potable water heating. 2.2 Exclusion. This standard does not apply to desupereater/water heaters supplied as components of factory assembled refrigeration or air conditioning units. SECTION 3 DEFINITIONS 3.1 Desuperheater/water heater. A factory- ...
Heat exchanger

A heat exchanger is a device used to transfer heat between one or more fluids. The fluids may be separated by a solid wall to prevent mixing or they may be in direct contact. They are widely used in space heating, refrigeration, air conditioning, power stations, chemical plants, petrochemical plants, petroleum refineries, natural-gas processing, and sewage treatment. The classic example of a heat exchanger is found in an internal combustion engine in which a circulating fluid known as engine coolant flows through radiator coils and air flows past the coils, which cools the coolant and heats the incoming air.