* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Phase Changes
Insulated glazing wikipedia , lookup
Dynamic insulation wikipedia , lookup
Building insulation materials wikipedia , lookup
Vapor-compression refrigeration wikipedia , lookup
Solar water heating wikipedia , lookup
Solar air conditioning wikipedia , lookup
Heat exchanger wikipedia , lookup
Intercooler wikipedia , lookup
Thermoregulation wikipedia , lookup
R-value (insulation) wikipedia , lookup
Heat equation wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Cogeneration wikipedia , lookup
Thermal conduction wikipedia , lookup
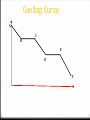
Table I 0 Exothermic reactions release heat and have negative values. 0 Example: When Carbon and Oxygen react they release 393.5kJ of heat per mole reacted. 0 Endothermic reactions absorb heat and have positive values. 0 Example: When Nitrogen and Oxygen react they absorb 182.6kJ of heat per mole. Table I examples 0 When 2 moles of CH4 burn in oxygen, how much heat is released? 0 When C2H4 is formed, is heat released or absorbed? 0 Reactions that release the most energy are the most stable. Which reaction becomes the most stable? 0 Where did these values come from? Calculating Heat of reactions 0 q is the symbol for heat. 0 If q is positive, the heat is endo. 0 If q is negative, the heat is exo. 0 q is measure in Joules, (J) or kilojoules (kJ). 0 The heat of a reaction is based on the mass of the substance, the temperature change it undergoes and specific heat. Specific Heat 0 Specific heat is the heat needed to raise the temperature of one gram of a substance one degree Celsius. To calculate heat: q = mcΔT 1. The temperature of 95.4g of copper increases from 25 to 48C and absorbed 849J. Calculate copper’s specific heat. Q= (95.4) (4.18) (48-25) Q = 0.387 J/gC q = mcΔT 2. How much heat is needed to raise the temperature of 100g of water 50C? Q = (100) (4.18) (50) Q = 20900J or 20.90 KJ q = mcΔT 3. If 600J are needed to heat 50g of water to 100C, what is the initial temperature? 600 = (50) (4.18) (x) X = 2.87 97.13C Kinetic Molecular Theory of Gases KMT describes perfect gases: 0 Gases move in constant, random, straight-line paths. 0 Gases are separated by large distances, much larger than their particle size. Therefore, gases volume is negligible. And gases are easily compressed. 0 Gases do not have attractive or repulsive forces between molecules. 0 Collisions between molecules can transfer energy but the total energy of the system is constant. This is called an elastic system. Kinetic Molecular Theory of Gases In summary, Perfect gases: 0 Have no mass 0 Have no volume 0 Have no intermolecular forces Kinetic Molecular Theory of Gases 0 But we don’t have perfect gases. How do real gases deviate from ideal gases? 0 They have a volume, mass and small IMF under high pressure and low temperature. 0 So, a real gas must be hot and under low pressure to behave like an ideal gas. Pressure 0 Gases exert a pressure on surrounding substances because they are constantly moving and colliding with other surfaces. 0 Only in a vacuum, where there are no molecules, there is no pressure. 0 Gas pressure can be measured in atmospheres or kilopascals, according to reference table A. Liquids 0 No definite shape 0 Definite volume 0 Constant motion 0 No arrangement 0 Molecules are closer together than a gas Solids 0 Definite shape 0 Definite volume 0 Constant vibration 0 Molecules are packed tightly in a geometric (crystalline) pattern Phase Changes Identify the phase change and if it’s endothermic or exothermic: Liquid to gas endothermic 0 Evaporation 0 Condensation Gas to liquid exothermic 0 Melting Solid to liquid endothermic 0 Freezing Liquid to solid exothermic 0 Sublimation Solid to gas endothermic 0 Deposition gas to solid exothermic Thermochemistry 0 The study of energy changes that occur in chemical reactions. 0 Kinetic energy refers to energy of motion. (Temperature) 0 Potential Energy refers to stored energy. Phase Change Diagrams Cooling Curve A C B E D F When can you use q=mcT? 0 Only on the solid, liquid and gas only lines. (Where the temperature changes) 0 So, what equations do we use if the temperature is not changing? Two more equations from Table T 0 Heat of vaporization: heat needed to change a substance from gas to liquid or liquid to gas. q=mHv 0 Heat of fusion: heat needed to change a substance from solid to liquid or liquid to solid. q=mHf 0 If the IMF is strong, the heats of vaporization and fusion is high. Q=mHv 1. Calculate the number of joules needed to vaporize 423g of H2O. Q = (423) (2260) 955, 980J or 955.98KJ Q=mHf 0 How much heat is needed to melt ice at 0C if the sample weighs 255g? Q = (255) (334) 85,170J or 85.17 KJ 0 When you finish # 1-4, try these: 5. How much heat is absorbed by 550g block of ice to raise the temperature from -15 to 0C? 6. How much heat energy must be absorbed to raise the temperature of a 200 gram block of ice from -10 to 0C and then completely melt it to a liquid at the same temperature? 7. How much energy would be required to heat the same 200g of liquid water in #6 (at 0C) to the normal boiling point of water and then vaporize it? 8. If the temperature of the 200 grams of steam generated in #7 were heated to a new temperature of 120C, how much energy would be absorbed? BONUS: What is the total amount of energy needed to heat 150g of ice at -10C to gas at 140C? (Use a heating curve to help you). Measuring heat in the lab You can measure the heat of physical and chemical changes in a calorimeter. The calorimeter acts like a styrofoam cup, it insulates the reaction (doesn’t let the overall heat change). Measuring heat in the lab 0 The heat released by the reaction equals the heat absorbed by the water. 0 You will measure the change in heat of the water using q=mcT. Measuring heat in the lab 0 You will use a calorimeter more like this. 0 You must make sure you always stir the solution to make the heat equal throughout the cup. A student places a 68.4g piece of metal at 99.5C in a calorimeter filled with 103g of water at 25.2C. The temperature changes to 27.6C. 1. 2. 3. 4. 5. In terms of the metal, is the reaction endothermic or exothermic? Calculate the heat absorbed by the water. Calculate the heat released by the metal. Calculate the specific heat of the metal. Using the following specific heats, determine the identity of the metal and calculate the % error. Aluminum: 0.21 J/gC Copper: 0.090 J/gC Gold: 0.030 J/gC 0 http://www.sciencephoto.com/media/233708/view