Homework #1: Energy Unit Conversions
... Solve the following problems using the conversions listed below. Report your answer with the correct units. Conversions: 1 calorie = 4.18 joules 1 Calorie = 1000 calories 1kilocalorie= 1 Calorie (food) 1. How many calories are in 16.00 joules? ...
... Solve the following problems using the conversions listed below. Report your answer with the correct units. Conversions: 1 calorie = 4.18 joules 1 Calorie = 1000 calories 1kilocalorie= 1 Calorie (food) 1. How many calories are in 16.00 joules? ...
Chapters 19&20
... • Kelvin scale is defined by the temperature of the triple point of pure water • Triple point – set of pressure and temperature values at which solid, liquid, and gas phases can coexist • International convention: T of the triple point of water is ...
... • Kelvin scale is defined by the temperature of the triple point of pure water • Triple point – set of pressure and temperature values at which solid, liquid, and gas phases can coexist • International convention: T of the triple point of water is ...
Worksheet – Measuring Heat
... 4. If the temperature of 34.4 g of ethanol increases from 25.0oC to 78.8oC, how much heat has been absorbed by the ethanol? (4.52 x 103 J) 5. A 4.50g nugget of pure gold absorbed 276 J of heat. What was the final temperature of the gold if the initial temperature was 25.0oC? The specific heat of gol ...
... 4. If the temperature of 34.4 g of ethanol increases from 25.0oC to 78.8oC, how much heat has been absorbed by the ethanol? (4.52 x 103 J) 5. A 4.50g nugget of pure gold absorbed 276 J of heat. What was the final temperature of the gold if the initial temperature was 25.0oC? The specific heat of gol ...
Chapter 11 Notes
... Thermochemistry • This chapter deals with studying heat changes that occur during chemical reactions, which is defined as thermochemistry. ...
... Thermochemistry • This chapter deals with studying heat changes that occur during chemical reactions, which is defined as thermochemistry. ...
Lab 1
... change T. However, when a substance undergoes a change of phase, e.g., solid to liquid or liquid to gas, the heat energy goes into doing work against the intermolecular forces and is not reflected in a change in the temperature of the substance. This heat energy is called the latent heat of fusion ...
... change T. However, when a substance undergoes a change of phase, e.g., solid to liquid or liquid to gas, the heat energy goes into doing work against the intermolecular forces and is not reflected in a change in the temperature of the substance. This heat energy is called the latent heat of fusion ...
$doc.title
... Then we study heat engines, the wording of the second law of thermodynamics, and the efficiency of heat engines and refrigerators, before moving on to the Carnot cycle. From both the practical and theoretical perspective, the Carnot cycle is of great importance as a heat engine operating with this i ...
... Then we study heat engines, the wording of the second law of thermodynamics, and the efficiency of heat engines and refrigerators, before moving on to the Carnot cycle. From both the practical and theoretical perspective, the Carnot cycle is of great importance as a heat engine operating with this i ...
Exam 3 review - Iowa State University
... 22. Determine whether each of the following statements is true or false. If false, modify the statement to make it true. a. An exothermic reaction is always spontaneous. b. When ΔG° is positive, the reaction cannot occur under any conditions. c. ΔS° is positive of a reaction in which there is an inc ...
... 22. Determine whether each of the following statements is true or false. If false, modify the statement to make it true. a. An exothermic reaction is always spontaneous. b. When ΔG° is positive, the reaction cannot occur under any conditions. c. ΔS° is positive of a reaction in which there is an inc ...
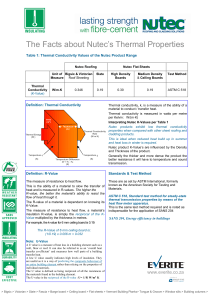
The Facts about Nutec Thermal Properties 11 5 12.pub
... thermal transmission properties by means of the heat flow meter apparatus. This is the same test method required and is noted as indispensable for the application of SANS 204 ...
... thermal transmission properties by means of the heat flow meter apparatus. This is the same test method required and is noted as indispensable for the application of SANS 204 ...
Name - Net Start Class
... 1. What is the relationship between the rate of cooling of a substance and the specific heat of that substance? The higher the specific heat number, the slower it is to cool (or heat up) 2. What is the formula for calculating heat? ...
... 1. What is the relationship between the rate of cooling of a substance and the specific heat of that substance? The higher the specific heat number, the slower it is to cool (or heat up) 2. What is the formula for calculating heat? ...
Thermodynamics-d2
... To find the work done by the gas, find the area under each segment, remembering the sign convention. ...
... To find the work done by the gas, find the area under each segment, remembering the sign convention. ...
Specific Heat and Calculating Heat Absorbed - Varga
... It turns out that water has a much higher specific heat capacity than concrete does. The specific heat of concrete is 0.84 J/g°C, whereas the specific heat of water 4.184 J/g°C. If you have 1 kg of each substance at 0°C, which of them will take more energy to raise to a temperature of 50°C? ...
... It turns out that water has a much higher specific heat capacity than concrete does. The specific heat of concrete is 0.84 J/g°C, whereas the specific heat of water 4.184 J/g°C. If you have 1 kg of each substance at 0°C, which of them will take more energy to raise to a temperature of 50°C? ...
151c15
... in a cycle that does nothing other than take in heat from a source and perform an equivalent amount of work! => no 100% efficient heat engines! 151c15:8 ...
... in a cycle that does nothing other than take in heat from a source and perform an equivalent amount of work! => no 100% efficient heat engines! 151c15:8 ...
Heat Transfer There are three mechanisms for the transfer of heat
... heat production, how does the geotherm look like? If there’s nonzero net heat flow per unit area out of the slab, this heat must be generated internally in the slab. In that case: d2 t q(y + δy) − q(y) = δy(−k 2 ) = δyρH, dy where: H is the heat production rate per unit mass ρ is density Question: w ...
... heat production, how does the geotherm look like? If there’s nonzero net heat flow per unit area out of the slab, this heat must be generated internally in the slab. In that case: d2 t q(y + δy) − q(y) = δy(−k 2 ) = δyρH, dy where: H is the heat production rate per unit mass ρ is density Question: w ...
Specific Heat!
... • The lower the specific heat – the easier (faster) it is to heat up and cool down. • The higher the specific heat – the harder (slower) it is to heat up and cool down. • Which substance would lose its heat fastest? • Which substance would take longer to heat up? ...
... • The lower the specific heat – the easier (faster) it is to heat up and cool down. • The higher the specific heat – the harder (slower) it is to heat up and cool down. • Which substance would lose its heat fastest? • Which substance would take longer to heat up? ...
Thermodynamics–Honors
... System = the area or space we focus on Surroundings = everything else apart from the system ...
... System = the area or space we focus on Surroundings = everything else apart from the system ...
student powerpoint 3
... • Movement of an electrical impulse, such as through a neuron. • During exercise- not much because source of heat exchange is little because the body surface area in contact with solid objects is small. ...
... • Movement of an electrical impulse, such as through a neuron. • During exercise- not much because source of heat exchange is little because the body surface area in contact with solid objects is small. ...
Specific Heat of a Metal
... volume of water in the calorimeter instead of its mass? 2. A 22.50 g piece of an unknown metal is heated to 100oC then transferred quickly and without cooling into 100.0 mL of water at 20.0oC. The final temperature reached by the system is 26.0oC. a. Calculate the quantity of heat absorbed by the wa ...
... volume of water in the calorimeter instead of its mass? 2. A 22.50 g piece of an unknown metal is heated to 100oC then transferred quickly and without cooling into 100.0 mL of water at 20.0oC. The final temperature reached by the system is 26.0oC. a. Calculate the quantity of heat absorbed by the wa ...
ENVIRONMENT & ANIMAL HEALTH
... production and heat loss from the body are about the same. • When ambient temperatures drop below the TNZ, animals increase feed intake and reduce blood flow to body extremities. Mammals generate heat by shivering. Birds fluff their feathers to increase insulation space around their bodies. ...
... production and heat loss from the body are about the same. • When ambient temperatures drop below the TNZ, animals increase feed intake and reduce blood flow to body extremities. Mammals generate heat by shivering. Birds fluff their feathers to increase insulation space around their bodies. ...
Joule`s Law and Heat Transfer Name:
... 7. Open DataStudio, select "Open Activity", select "Library", select "Physics Labs folder", and select "P16-Temperature and Heat". Click on the digits display and click start. 8. Plug in the power, and stir the water gently with the temperature sensor. 9. When the temperature reaches 20oC, the PC wi ...
... 7. Open DataStudio, select "Open Activity", select "Library", select "Physics Labs folder", and select "P16-Temperature and Heat". Click on the digits display and click start. 8. Plug in the power, and stir the water gently with the temperature sensor. 9. When the temperature reaches 20oC, the PC wi ...
Heat exchanger

A heat exchanger is a device used to transfer heat between one or more fluids. The fluids may be separated by a solid wall to prevent mixing or they may be in direct contact. They are widely used in space heating, refrigeration, air conditioning, power stations, chemical plants, petrochemical plants, petroleum refineries, natural-gas processing, and sewage treatment. The classic example of a heat exchanger is found in an internal combustion engine in which a circulating fluid known as engine coolant flows through radiator coils and air flows past the coils, which cools the coolant and heats the incoming air.