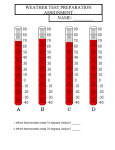
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Specific Heat and Calculating Heat Absorbed - Varga
Survey
Document related concepts
Space Shuttle thermal protection system wikipedia , lookup
Thermal conductivity wikipedia , lookup
Radiator (engine cooling) wikipedia , lookup
Underfloor heating wikipedia , lookup
Building insulation materials wikipedia , lookup
Hypothermia wikipedia , lookup
Dynamic insulation wikipedia , lookup
Solar water heating wikipedia , lookup
Heat exchanger wikipedia , lookup
Solar air conditioning wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Intercooler wikipedia , lookup
Cogeneration wikipedia , lookup
R-value (insulation) wikipedia , lookup
Heat equation wikipedia , lookup
Thermoregulation wikipedia , lookup
Transcript
Recall the definition of a calorie: The amount of energy required to raise the temperature of 1 gram of pure water by 1 degree Celsius. Also recall that the calorie is equal to 4.184 J. That quantity, 4.184 J/g°C, is known as the specific heat of water. The symbol for specific heat is (c). The specific heat of any substance is the amount of heat required to raise one gram of the substance by one degree Celsius. To raise the temperature of water by 1 degree Celsius requires 4.184 J of energy. You will find that other substances require much less energy to raise their temperatures by 1 degree Celsius. It turns out that water has a much higher specific heat capacity than concrete does. The specific heat of concrete is 0.84 J/g°C, whereas the specific heat of water 4.184 J/g°C. If you have 1 kg of each substance at 0°C, which of them will take more energy to raise to a temperature of 50°C? It takes more energy to heat the water to 50 degrees Celsius than it takes to heat the concrete block to 50 degrees Celsius! It seems like if we know the specific heats of many substances, we should be able to tell how much heat is required to raise the substances to a certain temperature. And guess what? We can! It turns out that three important things matter when calculating the heat lost or gained by a substance: Mass There is a direct relationship between mass and the amount of heat required to raise a substance to a specific temperature. The symbol for mass is m. In other words, the relationship between mass and heat required is linear. Temperature Change The temperature that you want to raise a substance to is equally important as the mass of the substance that you have. Temperature change is often given the symbol Δt, and is equal to final temperature – initial temperature. Specific Heat The specific heat of the substance is important because it tells you exactly how much heat is required to raise the temperature of the substance. The symbol for specific heat is (c). These 3 important factors lead us to the heat equation: q = mc Δt Where: q = heat (energy in J) m = mass (in grams) c = specific heat (in J/g°C) Δt = temperature change (in °C) 1. 12 g of ethanol were heated from an initial temperature 6.2 °C to a final temperature of 19.8 °C. How much heat was absorbed by the ethanol? 2. The temperature of a sample of iron with a mass of 10.0 g changed from 50.4 °C to 25.0 °C with the release of 114 J. What is the specific heat of iron?